Description
Details
Product Description
Human Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs) are multipotent mesenchymal stem/stromal cells that are capable of differentiating into the cells of the connective tissue in vitro, such as chondrocytes (cartilage), osteoblasts (bone), and adipocytes (fat). Cultured MSCs have shown promising applications in cell-based therapeutic strategies for specific tissue regeneration, immunomodulation, and cancer research.
StemExpress® Human BM-MSCs are obtained from whole bone marrow collected from the iliac crest. Bone marrow mononuclear cells are separated from whole bone marrow by a density gradient centrifugation protocol and subsequently cultured for 9 to 14 days in medium that promotes MSC propagation. Adherent cells are checked for the expression of specific MSC markers, including the expression of CD73, CD90, and CD105, and negative for the expression of CD14, CD34, CD45, and HLA-DR. Cultured bone marrow mesenchymal stromal/stem cells have a fibroblast-like morphology.
Sample Data
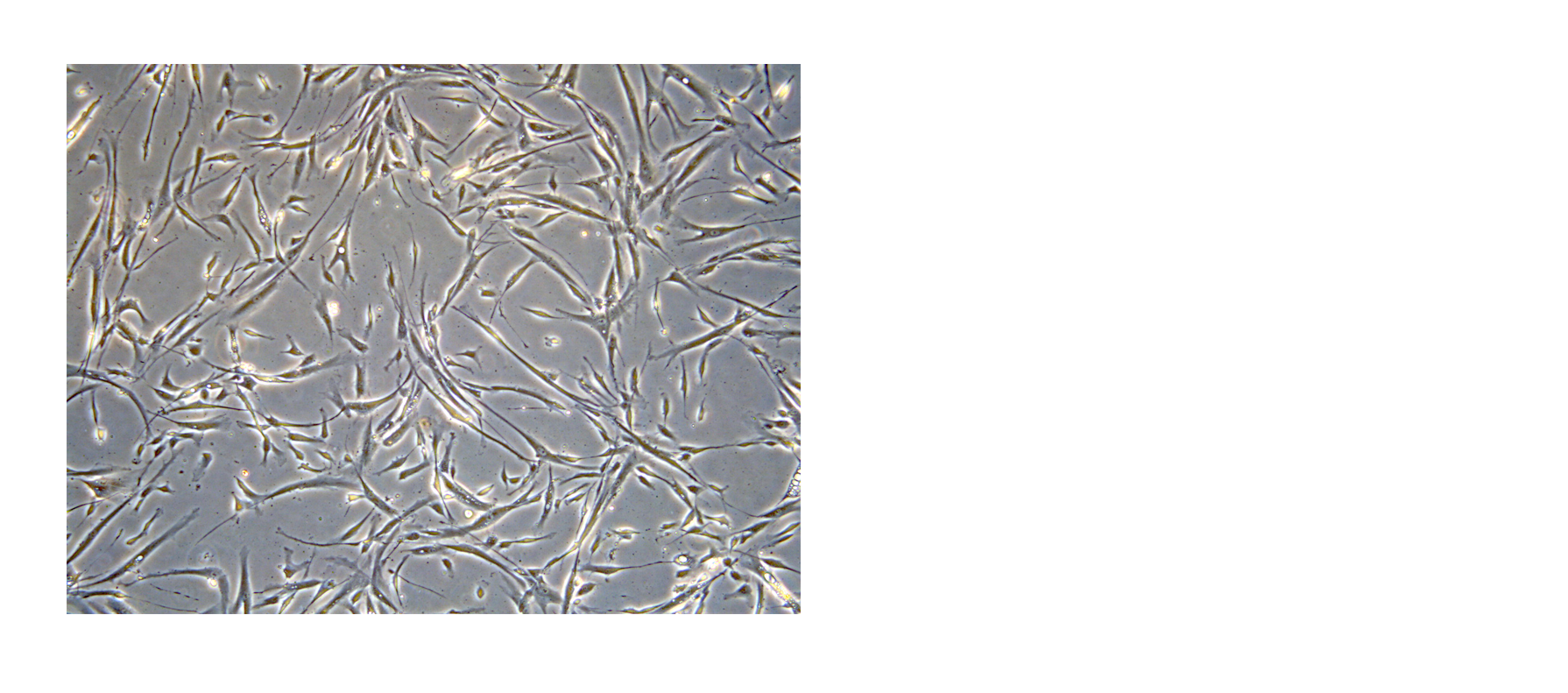
Figure 1. BM-MSCs cultured in NutriStem® MSC Medium supplemented with 5% PLTGold® Human Platelet Lysate show healthy, spindle-shaped morphology in culture.
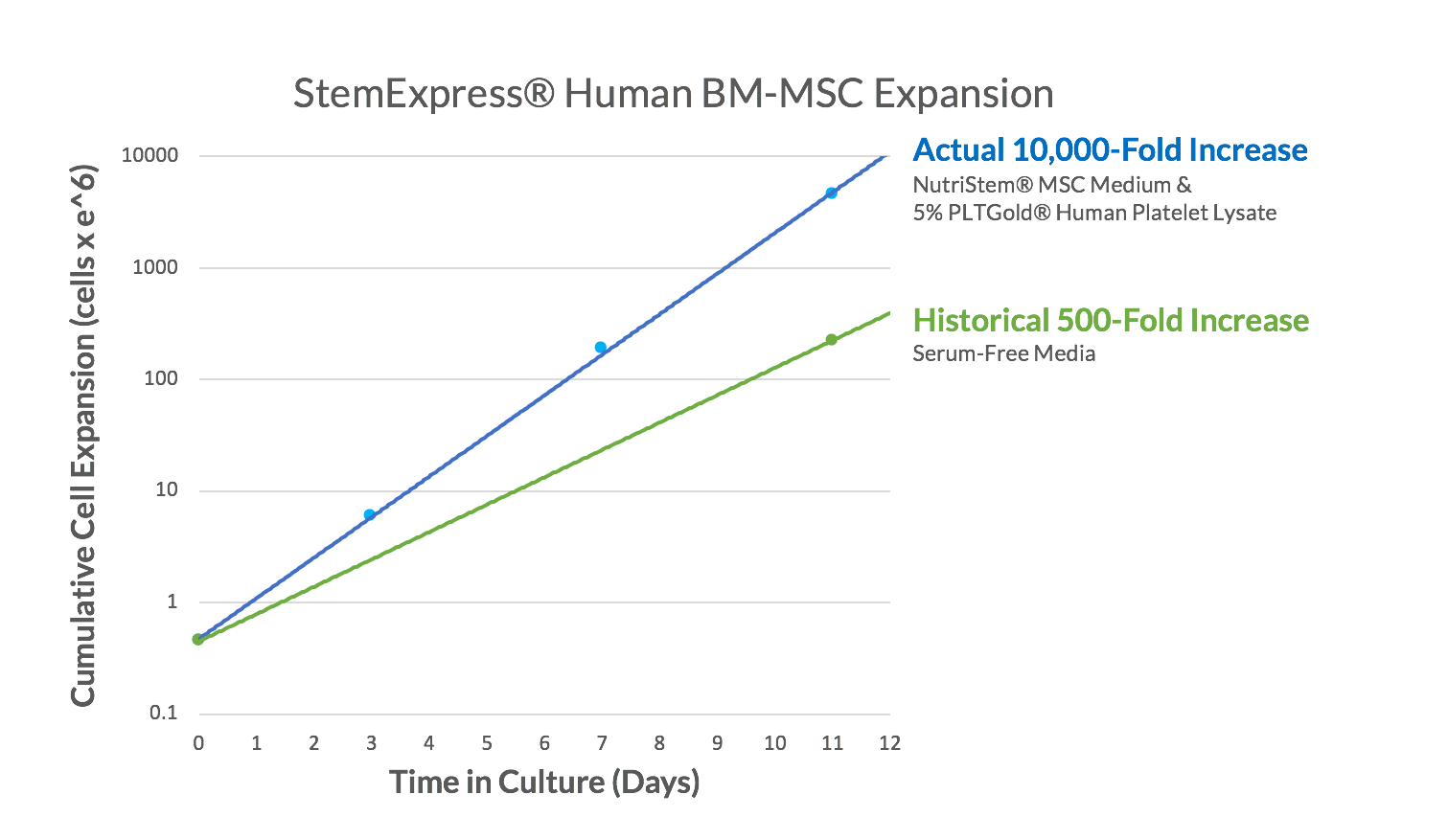 Figure 2. StemExpress® BM-MSCs show exceptional growth and proliferation when cultured in NutriStem® MSC Medium supplemented human platelet lysate. BM-MSCs were thawed directly into xeno-free, serum-free NutriStem® MSC Medium and 5% PLTGold® Human pPatelet Lysate and passaged every 3 to 4 days as the cultures reached 80% confluency. By Day 11, the cultures showed a 10,000-fold increase in cell number, much higher than the expected 500-fold expansion typically seen when the same cells are cultured in other serum-free media.
Figure 2. StemExpress® BM-MSCs show exceptional growth and proliferation when cultured in NutriStem® MSC Medium supplemented human platelet lysate. BM-MSCs were thawed directly into xeno-free, serum-free NutriStem® MSC Medium and 5% PLTGold® Human pPatelet Lysate and passaged every 3 to 4 days as the cultures reached 80% confluency. By Day 11, the cultures showed a 10,000-fold increase in cell number, much higher than the expected 500-fold expansion typically seen when the same cells are cultured in other serum-free media.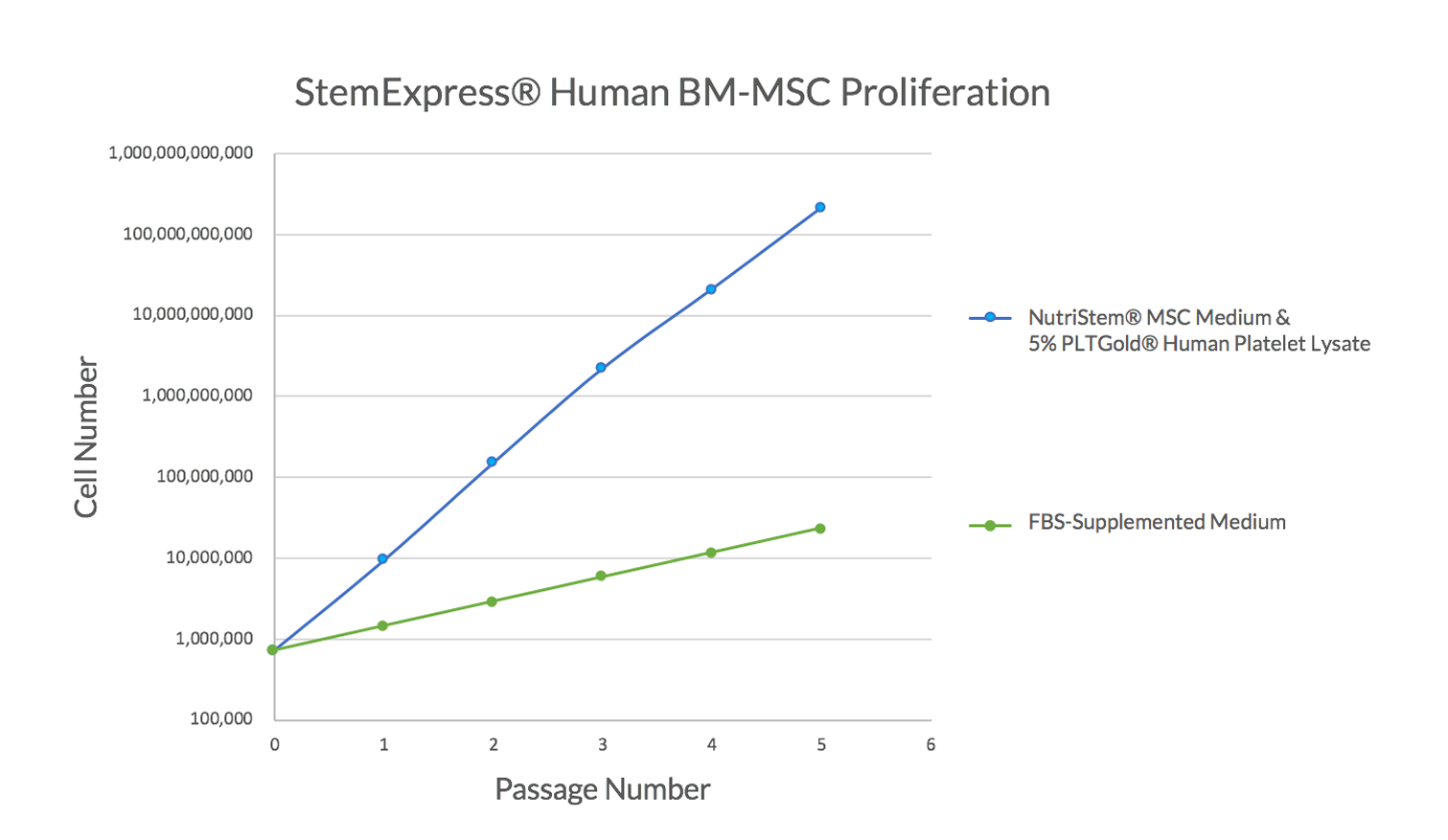 Figure 3. Proliferation of human BM-MSCs cultured in NutriStem® MSC Medium and human platelet lysate. BM-MSCs cultured in xeno-free NutriStem® MSC Medium supplemented with 5% PLTGold® Human Platelet Lysate expand rapidly in culture (passaged every 3 to 4 days), with high expansion rates compared to typical culture with serum-containing media (passaged every 7 days).
Figure 3. Proliferation of human BM-MSCs cultured in NutriStem® MSC Medium and human platelet lysate. BM-MSCs cultured in xeno-free NutriStem® MSC Medium supplemented with 5% PLTGold® Human Platelet Lysate expand rapidly in culture (passaged every 3 to 4 days), with high expansion rates compared to typical culture with serum-containing media (passaged every 7 days).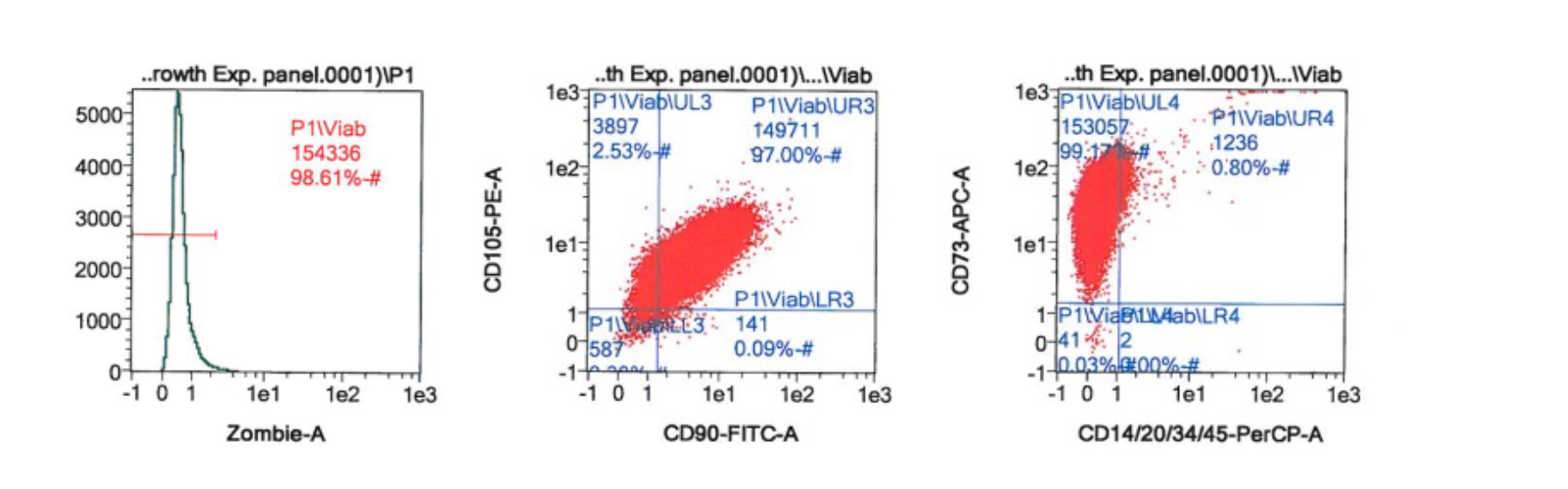
Figure 4. Each lot of StemExpress® Human BM-MSCs are validated by flow cytometry to ensure ≥85% viability (fresh) or ≥70% post-thaw viability (frozen). Cells are validated to show ≥90% positive expression of the MSC markers CD73, CD90, and CD105, and <5% expression of the negative markers CD14, CD34, and CD45.
Specifications
Specifications
| Species | Human |
|---|---|
| Disease State | Healthy |
| Cell and Tissue Source | Bone Marrow |
| Purity | >90% expression of CD73, CD90, and CD105 <5% expression of CD14, CD34, and CD45 Determined by flow cytometry |
| Viability | Fresh: ≥85% viability determined by flow cytometry Frozen: ≥70% viability determined by flow cytometry |
| Culture Environment | Culture Human BM-MSCs in a humidified incubator at 37°C and 5% CO2 |
| Donor Attributes | HIV-, HepB-, HepC- |
| Viral Testing | Collected cells are screened for HIV, HBV/HCV, LCMV (by PCR), and HLA-A2+ (by immunophenotype) |
| Derivation | Whole bone marrow is collected from the posterior iliac crest into a syringe containing heparin (anticoagulant). Mononuclear cells are enriched from the bone marrow suspension by a density gradient centrifugation protocol and subsequently cultured for 9 to 14 days in medium that promotes MSC propagation. After culture, adherent cells are checked by flow cytometry for >90% expression of positive and <5% of negative MSC markers. |
| Cryopreservation | Frozen StemExpress® Human BM-MSCs are carefully cryopreserved in a medium containing 10% DMSO as a cryoprotective agent. Cells are cryopreserved at a controlled-rate of 1°C per minute to -80°C and transferred to liquid nitrogen to ensure maximum viability. |
| Specifications | Safety: This product contains human biological material and must be handled at Biosafety Level 2 or higher. All biological products should be treated as potentially infectious or contaminated material, even if infectious disease screening reports are negative. Follow universal precautions and wear appropriate personal protective equipment. Preparation: Refer to appropriate counting, thawing, and passaging protocols for detailed culture instructions. Warranty: StemExpress warrantees its BM-MSCs to meet specifications for viability, purity, and cell count, provided instructions are followed exactly, and cells are tested immediately upon receipt (if fresh) or thaw (if frozen). StemExpress is not able to guarantee cell performance for any in vitro or in vivo culture system, proliferation assay, functional assay, or implantation. |
| Storage and Stability | Cells must be stored (if frozen) or processed (if fresh) immediately upon receipt. Fresh: Fresh cells must be processed immediately upon receipt, including cell counting and viability assessment following protocol. Cells should be cultured according to appropriate MSC protocols in a humidified incubator at 37°C and 5% CO2. Frozen: Cryopreserved cells are shipped within the US on dry ice. Immediately upon receipt, cells must be stored and maintained at -135°C or below (liquid nitrogen). Cells are validated to be stable when stored at -135°C or below for 1 year from date of receipt. |
| Instructions for Use | Cells are intended for in vitro diagnostic use only. Cells are not approved for human or veterinary use in vivo, diagnostic, or clinical applications. |
| Legal | Human BM-MSCs are obtained using StemExpress’ IRB-approved consent forms and protocols. |
Documentation
Materials Safety Data Sheet
Manuals & Protocols
 Thawing and Measuring Viability of Frozen BM-MSCs
Thawing and Measuring Viability of Frozen BM-MSCs Measuring Viability and Plating Fresh BM-MSCs
Measuring Viability and Plating Fresh BM-MSCs Passaging MSCs using NutriStem® MSC Medium and Human Platelet Lysate
Passaging MSCs using NutriStem® MSC Medium and Human Platelet Lysate Thawing Human MSCs in NutriStem® MSC Medium and Human Platelet Lysate
Thawing Human MSCs in NutriStem® MSC Medium and Human Platelet Lysate Cryopreservation of Human MSCs
Cryopreservation of Human MSCs
Product Literature
 StemExpress® Human BM-MSCs
StemExpress® Human BM-MSCs Application Note: Growing more cells: cGMP culture environments for high yield expansion of MSCs
Application Note: Growing more cells: cGMP culture environments for high yield expansion of MSCs
Certificate of Analysis
COA's can be downloaded from Sartorius's Certificates Portal.
For certificates issued before November 15, 2021, please enter below the product lot number and click search.






