Description
Details
Product Overview:
The EZ-PCR™ Mycoplasma Detection Kit is a highly-sensitive and -specific PCR assay designed to test for the presence of over 90 species of Mycoplasma, Acholeplasma, and Spiroplasma in cell cultures with a detection limit of 10 CFU/mL. The kit contains a ready-to-use PCR reaction mix with highly-optimized mycoplasma-specific primers, a positive control, and an internal control, all for the simple and efficient discovery of mycoplasma contamination. Samples can be prepared in as little as 10 minutes and results can be easily obtained within just a few hours.
- Easy to use. A complete PCR reaction mix that contains primers, Taq polymerase, and dNTPs is included.
- Easy to run. Follow a simple protocol with ready-to-use reagents for as little as 10 minutes of preparation.
- Easy to interpret. Internal and positive controls are included to ensure efficient and accurate PCR interpretation.
Avoid False Negatives - Internal Control Included
The EZ-PCR™ Mycoplasma Detection Kit includes an internal amplification control, a plasmid containing a non-mycoplasma-specific DNA sequence to test for false negatives. This internal control is simultaneously amplified in the tube with cell culture samples, and should be used with all PCR samples, including the negative and positive controls. This combination rules out inhibition from biological material, among other assay malfunctions that lead to misinterpretation of results, namely, false negatives.
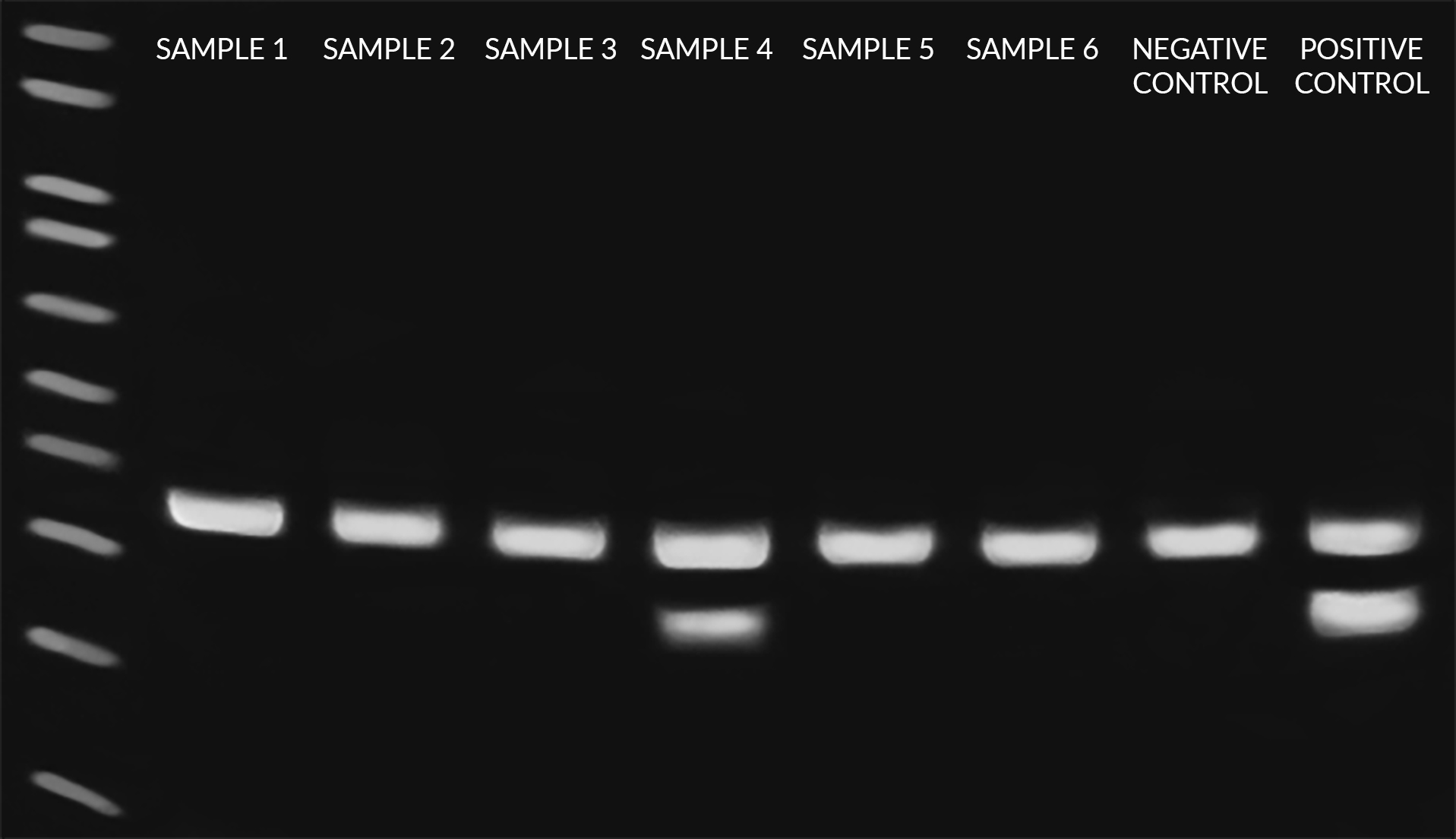
Figure 1: Gel electrophoresis results obtained following PCR reaction preparation and amplification using the EZ-PCR™ Mycoplasma Detection Kit. Eight total reactions, including six samples, one negative control, and one positive control were tested, each of which contain the internal control to rule out PCR inhibition (i.e. false negatives). As shown, Sample 4 produced a mycoplasma-positive band at 270bp in addition to the internal control band at 357bp, while Samples 1, 2, 3, 5, and 6 produced only internal control bands and are thus negative for mycoplasma. Image courtesy of WiCell Research Institute.
The Importance of Routine Testing
Mycoplasma are one of the most common, yet elusive, contaminants of mammalian cell cultures. As the smallest known free-living organism, mycoplasma are a pervasive, parasitic species of highly-infectious bacteria that are estimated to contaminate between 15-35% of all continuous cell cultures worldwide. While other mycoplasma detection methods are available, PCR-based assays have the highest sensitivity with minimal preparation time for early detection in a rapid and simple manner, when compared to other methods.The earlier mycoplasma contamination is discovered, the simpler it is to treat. The easy-to-use and cost-efficient EZ-PCR™ Mycoplasma Detection Kit was designed for highly-sensitive routine screening and detection of mycoplasma and other closely related species. To avoid major contamination, testing should be carried out minimally every 2 weeks to 3 months, especially when shared incubator spaces are used, as well as prior to the incorporation of new cultures from outside sources.
Efficient and precise PCR-based testing for mycoplasma infection should also be conducted throughout the cell culture manufacturing process from inoculation through harvest, involving routine tests of raw materials, cell banks, and viral seed stocks.
 Figure 2. Images represent examples of mycoplasma that are capable of infecting all cell culture labs. Mycoplasma can be self-replicating and are the smallest known free-living prokaryote. Mycoplasma contamination can not be seen under a standard microscopes.
Figure 2. Images represent examples of mycoplasma that are capable of infecting all cell culture labs. Mycoplasma can be self-replicating and are the smallest known free-living prokaryote. Mycoplasma contamination can not be seen under a standard microscopes.
Requirements from Leading Scientific Journals and European Pharmacopoeia Guidance
Due to the prevalence of mycoplasma in cell cultures and, more importantly, the adverse effects on a number of cellular functions that contamination causes, many major, high-impact scientific journals have begun requiring proof of testing prior to publication. Moreover, regulatory guidance recommends that all products derived from mammalian cell culture be tested for the presence of mycoplasma. Already in 2007, the European Pharmacopoeia (5.8, Sec. 2.6.7) provided guidance on the validation requirements for nucleic acid amplification–based methods (PCR) as a substitute for the time-consuming, direct cell culture methods of detection.Specifications
Specifications
| QTY | 20 reactions |
|---|---|
| Brand | EZ-PCR™ |
| Storage Conditions | -20º |
| Shipping Conditions | Dry Ice |
References
references
- L. Tao et al. Natural pentacyclic triterpenoid from Pristimerin sensitizes p53-deficient tumor to PARP inhibitor by ubiquitination of Chk1. Pharmacological Research, volume 201, March 2024
- A. Stern et al. Inferring mitochondrial and cytosolic metabolism by coupling isotope tracing and deconvolution. Nature Communications, 2023
- M. E. Silva et al. DDR1 and Its Ligand, Collagen IV, Are Involved in In Vitro Oligodendrocyte Maturation. Int. J. Mol. Sci. 2023
- H. Yamazaki et al. Hypoxia-targeting therapy for intestinal T-cell lymphoma in dogs: Preclinical study using 3D in vitro models. Veterinary and Comparative Oncology, 2022
- Y. Yung et al. target="_blank">Progranulin, a moderator of estrogen/estrogen receptor α binding, regulates bone homeostasis through PERK/p-eIF2 signaling pathway. Journal of Molecular Medicine volume 100, pages1191–1207 (2022)
- S. Cao et al. Human induced pluripotent stem cells generated from a 45-years-old male donor of type 2 diabetes mellitus with APOE-epsilon3/epsilon3 alleles. Stem Cell Research, volume 63, 2022
- TC Wu et al. Identification of distinct slow mode of reversible adaptation of pancreatic ductal adenocarcinoma to the prolonged acidic pH microenvironment Journal of Experimental & Clinical Cancer Research volume 41, Article number: 137 (2022)
- E. Kahraman, E Göker, Anticancer effects of imidazole nucleus in hepatocellular carcinoma cell lines via the inhibition of AKT and ERK1/2 signaling pathways. Molecular Biology Reports, 2022
- N. C. Hettige, et al. FOXG1 dose tunes cell proliferation dynamics in human forebrain progenitor cells. Stem Cell Reports, 2022, https://doi.org/10.1016/j.stemcr.2022.01.010.
- E. Vulpis, et al. Impact on NK cell functions of acute versus chronic exposure to extracellular vesicle-associated MICA: Dual role in cancer immunosurveillance. Journal of Extracellular Vesicles, 2022. https://doi.org/10.1002/jev2.12176
- X. Jiani et al. Hypomethylation-activated cancer-testis gene LIN28B promotes cell proliferation and metastasis in gastric cancer. Gene. 2022, https://doi.org/10.1016/j.gene.2021.146115.
- L. Hung-Yu, et al. Epigenetic therapy combination of UNC0638 and CI-994 suppresses breast cancer via epigenetic remodeling of BIRC5 and GADD45A. Biomedicine & Pharmacotherapy, 2022, https://doi.org/10.1016/j.biopha.2021.112431.
- J. You, et al. Generation of a homozygous LRPAP1 knockout human embryonic stem cell line (FDCHDPe009-B) by CRISPR/Cas9 system. Stem Cell Research, 2021, https://doi.org/10.1016/j.scr.2021.102516.
- M. Rosner & M. Hengstschläger, Three-dimensional migration of human amniotic fluid stem cells involves mesenchymal and amoeboid modes and is regulated by mTORC1. Stem Cells, 2021. DOI: 10.1002/stem.3441
- L. Kampel, et al. Therapeutic inhibitory RNA in head and neck cancer via functional targeted lipid nanoparticles, Journal of Controlled Release (2021), https://doi.org/10.1016/j.jconrel.2021.07.034
- A. Salhab, et al. Sodium+/taurocholate cotransporting polypeptide as target therapy for liver fibrosis. Gut, 15 July 2021. doi: 10.1136/gutjnl-2020-323345
- L. Lavra, et al. Generation and characterization of the human induced pluripotent stem cell (hiPSC) line NCUFi001-A from a patient carrying KCNQ1 G314S mutation. Stem Cell Research, 2021, https://doi.org/10.1016/j.scr.2021.102418.
- R. M. Dulay, et al. Cytotoxicity of Gymnopilus purpureosquamulosus extracts on hematologic malignant cells through activation of the SAPK/JNK signaling pathway. (2021) PLoS ONE 16(5): e0252541. https://doi.org/10.1371/journal.pone.0252541
- A. Danilevsky, et al. Real-time selective sequencing using nanopores and deep learning. Research Square, 2021 https://doi.org/10.21203/rs.3.rs-540693/v1
- X. Gao, et. al. Generation of an iPSC line (GWCMCi002-A) from an X-linked Alport syndrome patient with a hemizygous splicing mutation (NM_000495.4, c. 1517-1 G > T) in the COL4A5 gene. Stem Cell Research, 2021, https://doi.org/10.1016/j.scr.2021.102388.
- R. Guo, et. al. Reprogramming of a human induced pluripotent stem cell line from one 48-year-old healthy male donor. Stem Cell Research, Vol. 53, 2021, https://doi.org/10.1016/j.scr.2021.102339.
- X. Wang, et. al. Derivation of induced pluripotent stem cells from one child suffering Potocki-Lupski syndrome. Stem Cell Research, Volume 53, 2021, https://doi.org/10.1016/j.scr.2021.102324.
- T.W. Hsu, et. al. Transplantation of 3D MSC/HUVEC spheroids with neuroprotective and proangiogenic potentials ameliorates ischemic stroke brain injury. Biomaterials, Vol. 272, 2021, https://doi.org/10.1016/j.biomaterials.2021.120765.
- T.Y. Chang, et. al. A novel histone deacetylase inhibitor MPT0L184 dysregulates cell-cycle checkpoints and initiates unscheduled mitotic signaling. Biomedicine & Pharmacotherapy, 2021, https://doi.org/10.1016/j.biopha.2021.111485.
- S. Kim, et. al. Inhibition of platelet‑derived growth factor receptor synergistically increases the pharmacological effect of tamoxifen in estrogen receptor α positive breast cancer. Oncology Letters, (2021). 21, 294. https://doi.org/10.3892/ol.2021.12555
- C. B. Lanauze, et. al. Colorectal cancer-associated Smad4 R361 hotspot mutations boost Wnt/β-catenin signaling through enhanced Smad4-LEF1 binding, Mol Cancer Res February 19 2021 DOI: 10.1158/1541-7786.MCR-20-0721
- S. Xiaofang, et al. Generation of a gene-corrected isogenic iPSC line (AHQUi001-A-1) from a patient with familial hypertriglyceridemia (FHTG) carrying a heterozygous p.C310R (c.928 T > C) mutation in LPL gene using CRISPR/Cas9. Stem Cell Research, Volume 52, 2021, https://doi.org/10.1016/j.scr.2021.102230.
- I. Angel, et al. Tenascin C promotes cancer cell plasticity in mesenchymal glioblastoma. Oncogene (2020). https://doi.org/10.1038/s41388-020-01506-6
- L. Monteran, et al. Bone metastasis is associated with acquisition of mesenchymal phenotype and immune suppression in a model of spontaneous breast cancer metastasis. Scientific Reports Vol. 10, Iss. 1, (2020). DOI:10.1038/s41598-020-70788-3
- W. Wang, et al. Generation of a human induced pluripotent stem cell line PUMCi001-A from a patient with Krabbe disease. Stem Cell Research Volume 48, October 2020, https://doi.org/10.1016/j.scr.2020.101937
- S. Yuval, Method of Treating Cancer with a Cancer Therapy in Combination with Another Therapeutic Agent. 06/18/2020, US Patent App 16/712800.
- N.Komura, et al. The role of myeloid-derived suppressor cells in increasing cancer stem-like cells and promoting PD-L1 expression in epithelial ovarian cancer. Cancer Immunol Immunother (2020). https://doi.org/10.1007/s00262-020-02628-2
- C. Huang, et al.Irisin, an exercise myokine, potently suppresses tumor proliferation, invasion, and growth in glioma. The FASEB Journal. 2020;00:1–16, http://DOI:10.1096/fj.202000573RR
- B. Feng, et al. Induced pluripotent stem cell line derived from a sporadic amyotrophic lateral sclerosis patient. Stem Cell Research 8 May 2020, 101841, https://doi.org/10.1016/j.scr.2020.101841
- D. Rovina, et al. Generation of the Becker muscular dystrophy patient derived induced pluripotent stem cell line carrying the DMD splicing mutation c.1705-8 T>C. Stem Cell Research, 22 April 2020, https://doi.org/10.1016/j.scr.2020.101819
- García et al. Functional and genomic characterization of three novel cell lines derived from a metastatic gallbladder cancer tumor. Biol Res (2020) 53:13. https://doi.org/10.1186/s40659-020-00282-7
- E. A.Sagredo, et al. ADAR1 Transcriptome editing promotes breast cancer progression through the regulation of cell cycle and DNA damage response. (BBA) - Molecular Cell Research, 8 April 2020, 118716, https://doi.org/10.1016/j.bbamcr.2020.118716
- I. Yamaguchi, et al. High cell density increases glioblastoma cell viability under glucose deprivation via degradation of the cystine/glutamate transporter xCT (SLC7A11) .JBC, April 7, 2020, doi: 10.1074/jbc.RA119.012213
- Z. Chen et al. Generation of an iPSC line (SKLOi001-A) from a patient with CLCN2-related leukoencephalopathy Stem Cell Research, 17 March 2020, https://doi.org/10.1016/j.scr.2020.101769
- Y. Huo et al. Establishment of human induced pluripotent stem cell line from dermal fibroblasts of a 78-year-old healthy individual Stem Cell Research, 17 March 2020, https://doi.org/10.1016/j.scr.2020.101762
- C. Xiao, et al.Generation of an isogenic gene-corrected iPSC line (PUMCHi001-A-1) from a familial partial lipodystrophy type 2 (FPLD2) patient with a heterozygous R349W mutation in the LMNA gene Stem Cell Research 14 March 2020, 101753, https://doi.org/10.1016/j.scr.2020.101753
- E. Jankauskaitė, et al.Testosterone increases apoptotic cell death and decreases mitophagy in Leber’s hereditary optic neuropathy cells. J Appl Genetics (2020). https://doi.org/10.1007/s13353-020-00550-y
- V. Borgna, et al. Targeting antisense mitochondrial noncoding RNAs induces bladder cancer cell death and inhibition of tumor growth through reduction of survival and invasion factors. Journal of Cancer 2020, Vol. 11, doi: 10.7150/jca.38880
- S.Y. Wu and C.S. Chiang, Distinct Role of CD11b+Ly6G−Ly6C− Myeloid‐ Derived Cells on the Progression of the Primary Tumor and Therapy‐Associated Recurrent Brain Tumor Cells 2020, 9, 51; doi:10.3390/cells9010051
- S. Han, et al. Generation of human iPSC line from a patient with Tetralogy of Fallot, YAHKMUi001-A, carrying a mutation in TBX1 gene Stem Cell Research,12 December 2019, 101687, https://doi.org/10.1016/j.scr.2019.101687
- J. Ma, et al. Induced pluripotent stem cell (iPSC) line (HEBHMUi002-A) from a healthy female individual and neural differentiation Stem Cell Research, Volume 42, January 2020, 101669 https://doi.org/10.1016/j.scr.2019.101669
- C. Xiao, et al. Generation of an integration-free induced pluripotent stem cell line (PUMCHi001-A) from a patient with familial partial lipodystrophy type 2 (FPLD2) carrying a heterozygous p.R349W (c.1045C>T) mutation in the LMNA gene Stem Cell Research, 6 November 2019, 101651, https://doi.org/10.1016/j.scr.2019.101651
- N. Veiga, et al. Leukocyte-Specific siRNA Delivery Revealing IRF8 as a Potential Anti-Inflammatory Target Journal of Controlled Release, 18 October 2019, https://doi.org/10.1016/j.jconrel.2019.10.001
- C. L. Liu, et al. Targeting the pentose phosphate pathway increases reactive oxygen species and induces apoptosis in thyroid cancer cells Molecular and Cellular Endocrinology, Volume 499, 1 January 2020, 110595, https://doi.org/10.1016/j.mce.2019.110595
- V. Alari, et al. Generation of three iPSC lines (IAIi002, IAIi004, IAIi003) from Rubinstein-Taybi syndrome 1 patients carrying CREBBP non sense c.4435G>T, p.(Gly1479*) and c.3474G>A, p.(Trp1158*) and missense c.4627G>T, p.(Asp1543Tyr) mutations Stem Cell Research, Volume 40, October 2019, 101553
- M. Mura et al., Generation of two human induced pluripotent stem cell (hiPSC) lines from a long QT syndrome South African founder population Stem Cell Research 24 July 2019, 101510 https://doi.org/10.1016/j.scr.2019.101510
- X. Bai et al. Generation of an integration-free induced pluripotent stem cell line (FDEENTi003-A) from a patient with pathological myopia Stem Cell Research, Volume 39, August 2019, 101495
- S. Kalaora et al. Combined Analysis of Antigen Presentation and T-cell Recognition Reveals Restricted Immune Responses in Melanoma Cancer Discov., 2018 November ; 8(11): 1366–1375. doi:10.1158/2159-8290.CD-17-1418.
- C.W.Q. Koh et al. Single-nucleotide-resolution sequencing of human N6-methyldeoxyadenosine reveals strand-asymmetric clusters associated with SSBP1 on the mitochondrial genome. Nucleic Acids Research, Volume 46, Issue 22, 14 December 2018, Pages 11659–11670
- A.Belostozky et al. Solidification of oil liquids by encapsulation within porous hollow silica microspheres of narrow size distribution for pharmaceutical and cosmetic applications. Materials Science and Engineering: C Volume 97, April 2019, Pages 760-767
- X. Bai et al. Establishment of an induced pluripotent stem cell line (FDEENTi001-A) from a patient with pathological myopia. Stem Cell Research, 2018
- E. Ruiz et al. Compositions for Protecting Skin Comprising DNA Repair Enzymes and Phycobiliprotein. US Patent App. 15/746,287, 2018
- M. Khoury et al. Treatment for Infection Composed of Menstrual Stem Cells US Patent App. 15/768,469, 2018
- A. Yoshimura et al. Exosomal miR-99a-5p is elevated in sera of ovarian cancer patients and promotes cancer cell invasion by increasing fibronectin and vitronectin expression in neighboring peritoneal mesothelial cells. BMC Cancer201818:1065
- K.L. Eales et al. Verteporfin selectively kills hypoxic glioma cells through iron-binding and increased production of reactive oxygen species. Scientific Reportsvolume 8, Article number: 14358 (2018)
- V. Alari et al. Generation of the Rubinstein-Taybi syndrome type 2 patient-derived induced pluripotent stem cell line (IAIi001-A) carrying the EP300 exon 23 stop mutation c.3829A > T, p.(Lys1277*), Stem Cell Research, Volume 30, July 2018, Pages 175-179
- S. Franceschi et al. Mitochondrial enzyme GLUD2 plays a critical role in glioblastoma progression. EBioMedicine, 2018
- M. Mura et al. Generation of the human induced pluripotent stem cell (hiPSC) line PSMi002-A from a patient affected by the Jervell and Lange-Nielsen syndrome and carrier of two compound heterozygous mutations on the KCNQ1 gene. Stem Cell Research, Volume 29, May 2018, Pages 157-161
- V. Marchan, et al. Towards novel angiogenesis inhibitors based on the conjugation of organometallic platinum(II) complexes to RGD peptides. ChemMedChem, 2018
- J. Khan et al. The protective effect of α-lipoic acid against bisphenol A-induced neurobehavioral toxicity. Neurochemistry International, 2018
- J. Rosati et al. Production and characterization of human induced pluripotent stem cells (iPSCs) from Joubert Syndrome: CSSi001-A (2850). Stem Cell Research, Volume 27, March 2018, Pages 74–77
- Gazit, et al. Recognition of Mycoplasma hyorhinis by CD99-Fc molecule. European Journal of Immunology, 34 (7): 2032-2040 (2004)
- Johansson, et al. Platelet Lysate: a Replacement for Fetal Bovine Serum in Animal Cell Culture, Cytotechnology 42 (2): 67-74 (2003)
- Epsztejn, et al. H-Ferritin Subunit Overexpression in Erythroid Cells Reduces the Oxidative Stress Response and Induces Multidrug Resistance Properties. Blood 94 (10): 3593-3603 (1999)
Documentation
Materials Safety Data Sheet
Manuals and Protocols
 EZ-PCR™ Mycoplasma Detection Kit Protocol (shipped March 2019 or later)
EZ-PCR™ Mycoplasma Detection Kit Protocol (shipped March 2019 or later) EZ-PCR™ Mycoplasma Detection Kit Instructions for Use (shipped March 2019 or later)
EZ-PCR™ Mycoplasma Detection Kit Instructions for Use (shipped March 2019 or later) EZ-PCR™ Mycoplasma Detection Kit without Internal Control Instructions for Use (shipped before March 2019)
EZ-PCR™ Mycoplasma Detection Kit without Internal Control Instructions for Use (shipped before March 2019)
Product Literature
Certificate of Analysis
COA's can be downloaded from Sartorius's Certificates Portal.
For certificates issued before November 15, 2021, please enter below the product lot number and click search.
Reviews
Customer Reviews (7)
- Top product!!!Review by Thomas G.
-
We use EZ-PCR™ Mycoplasma Detection Kit to test our iPS cells periodically of mycoplasma contamination and are absolutely excited about the manual, the handling, the procedure, etc. of it. It is very easy to use and you get reliable results very quickly in only a few steps. (Posted on 3/18/2020)Quality - Easy to use and fast Review by John-Mario Roussis
-
It's an easy and fast way to check whether your cell lines are contaminated with mycoplasma (Posted on 4/24/2018)Quality - easy to use , very efficientReview by amina jbara
-
Easy to use, very efficient. (Posted on 1/15/2018)Quality - very easy to useReview by Gidi Karmon
-
very easy to use, quick protocol, consistent results. (Posted on 1/11/2018)Quality - ExellentReview by Orit Sagi-Assif
-
Very good product, get quick results.Quality
The price is too high.
(Posted on 1/9/2018) - Good productReview by Sharon Tal
-
Very helpful. easy to use (Posted on 11/15/2017)Quality - GoodReview by Gk
-
Good (Posted on 10/27/2017)Quality





