Description
Details
Product Overview:
EndoGo™ XF Medium is a novel xeno-free culture medium specially designed for long-term expansion of large and small vessels endothelial cells from various sources. The medium provides optimally balanced nutritional environment that selectively promotes proliferation of normal human endothelial cells , while maintaining typical cobblestone-like cell morphology, phenotypic surface marker profile, and angiogenic differentiation potential.
EndoGo™ XF supports human macro and microvascular endothelial cells (MVEC).
Advantages
- Defined, xeno-free, serum-free medium
- Supports long-term expansion of large and small vessels endothelial cells
- Maintains high proliferation potential, typical morphology and EC marker expression
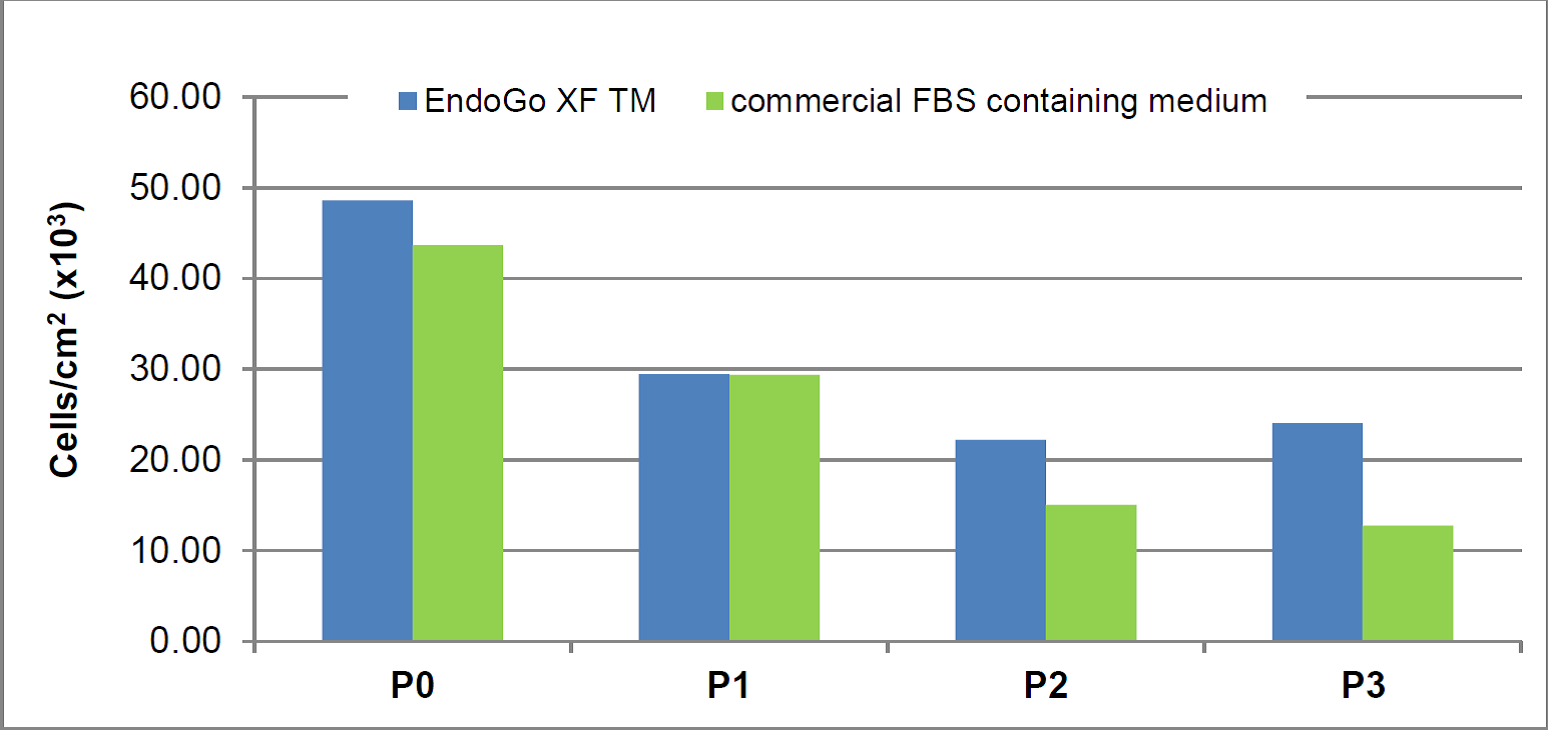
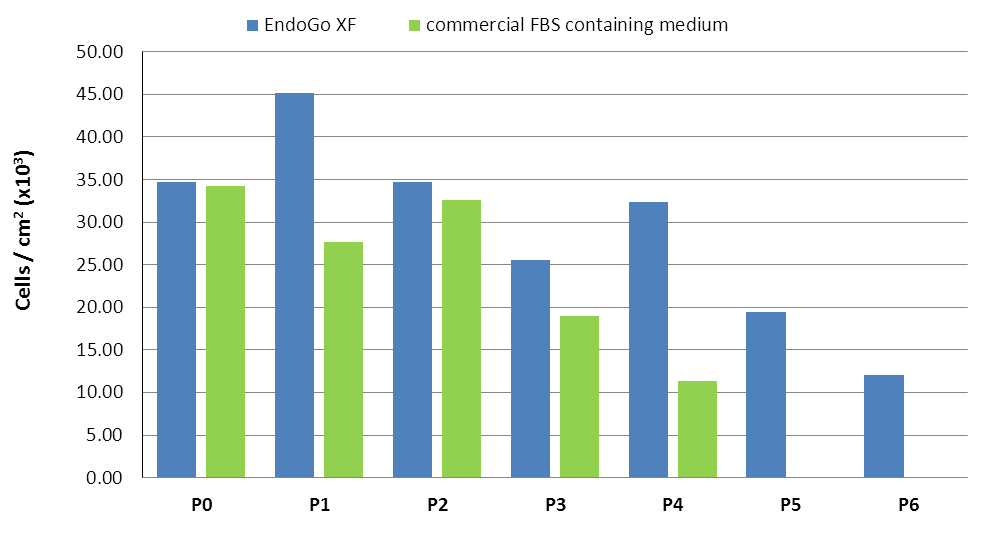
Cell counts and population doubling level (PDL) of HDMEC and HPMEC expanded for several passages in EndoGo™ XF in comparison to commercial FBS-containing medium. Viable cells were counted using ChemoMetec Viability and Cell Count Assay.
*HPMEC did not survive P5 in the FBS-containing medium.
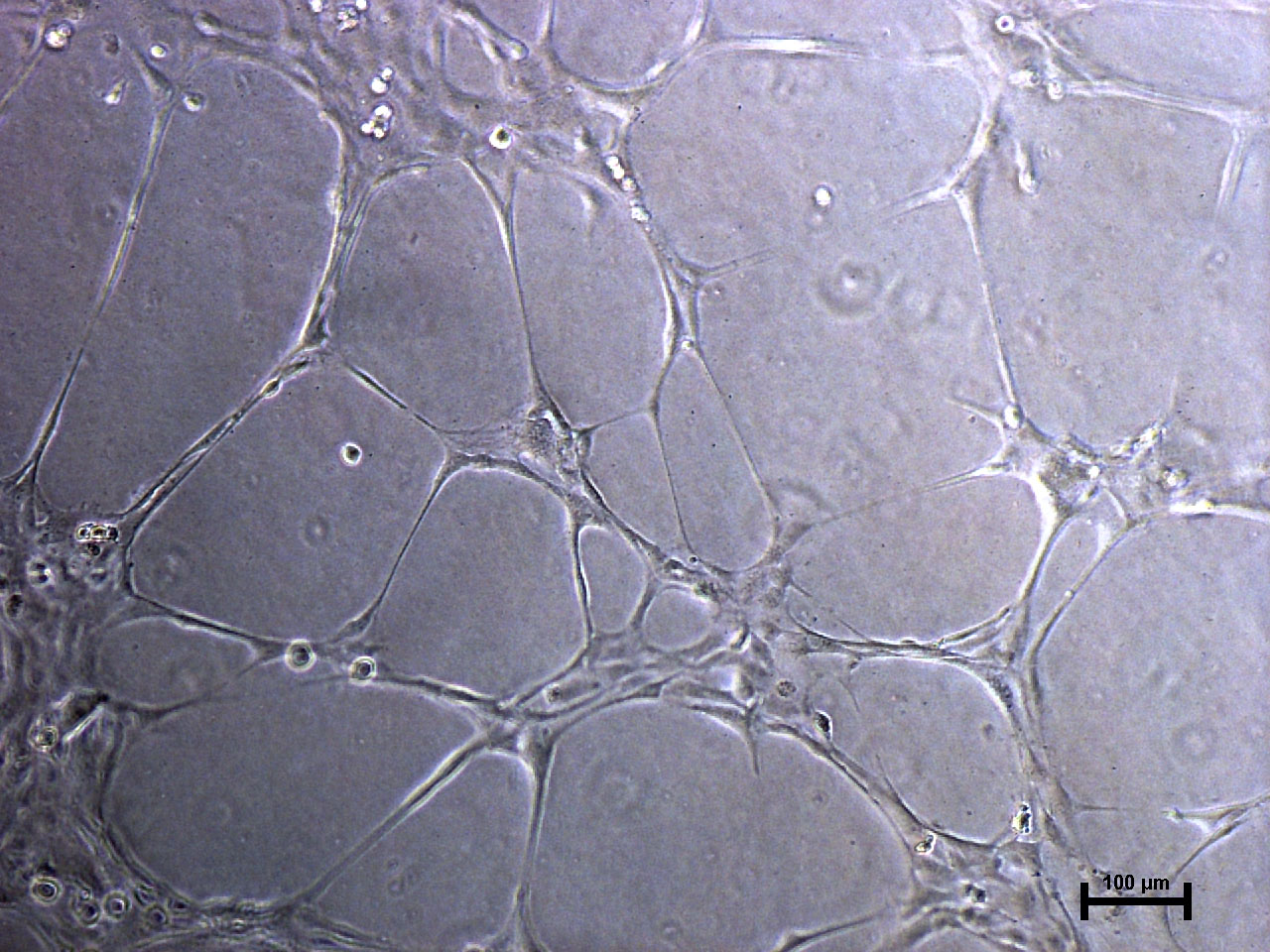
Macro vascular HUVEC (Human Umbilical Vein Endothelial Cells)
Micro vascular HDMEC (Human Dermal Microvascular Endothelial Cells)
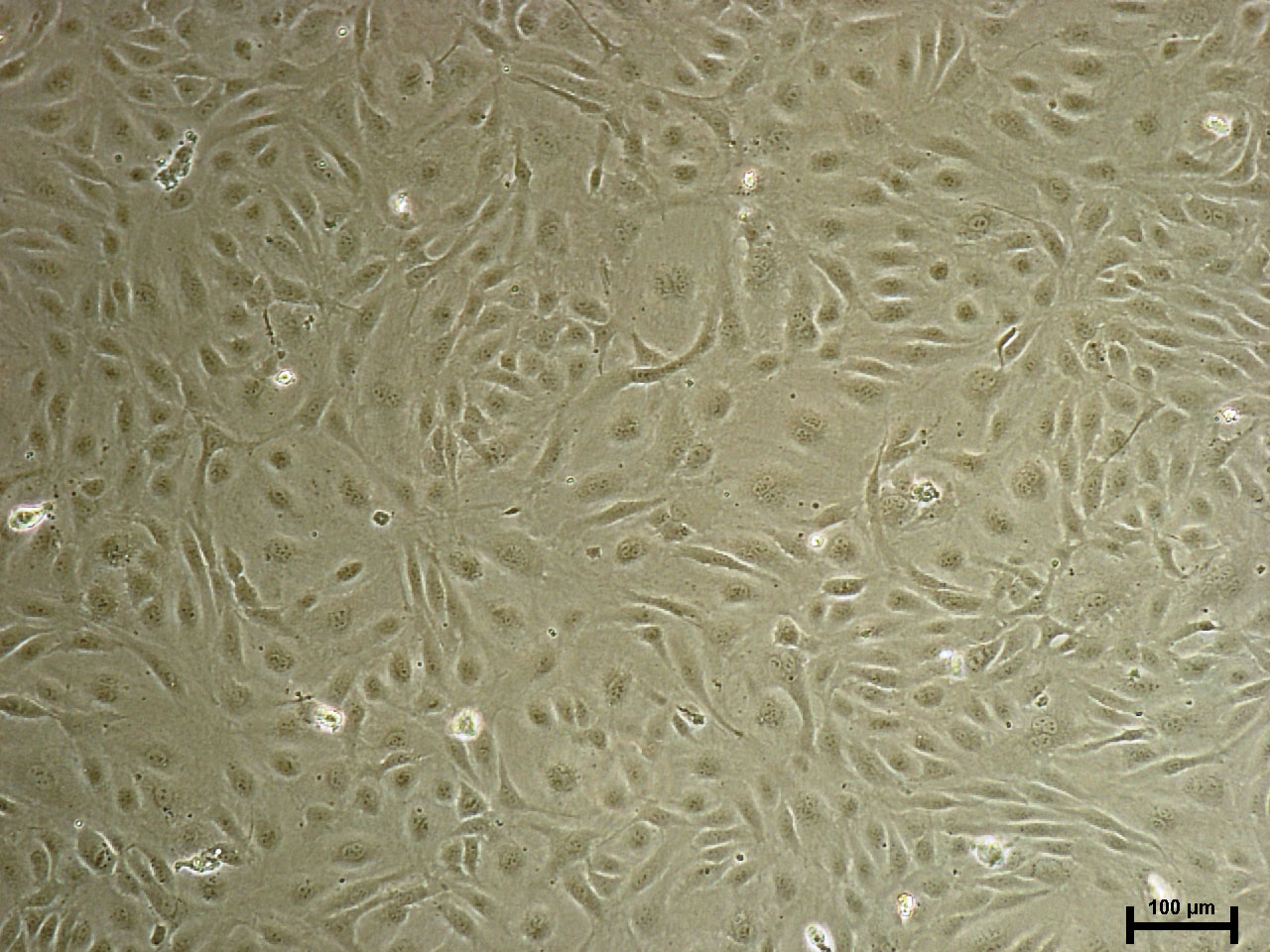
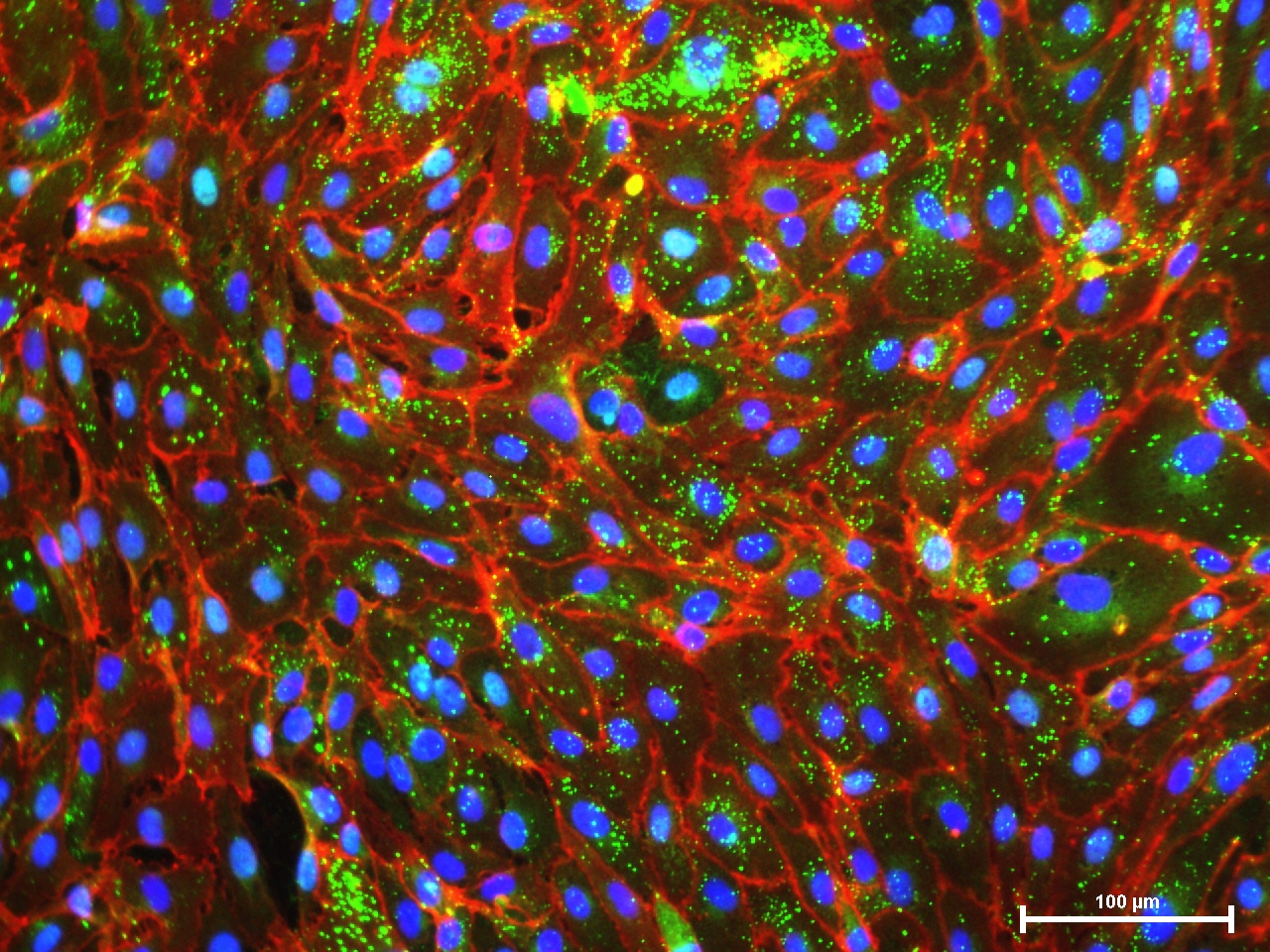
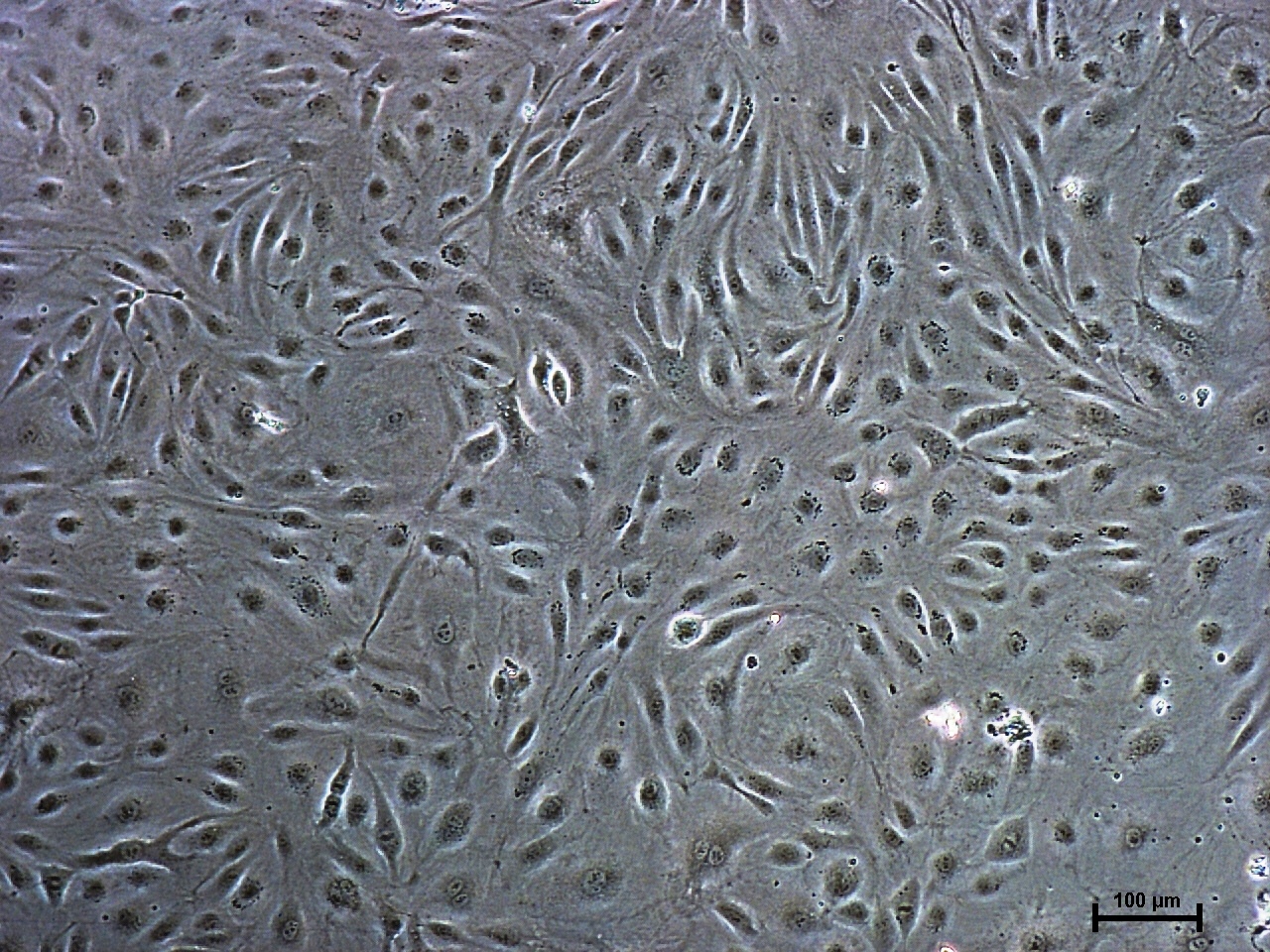
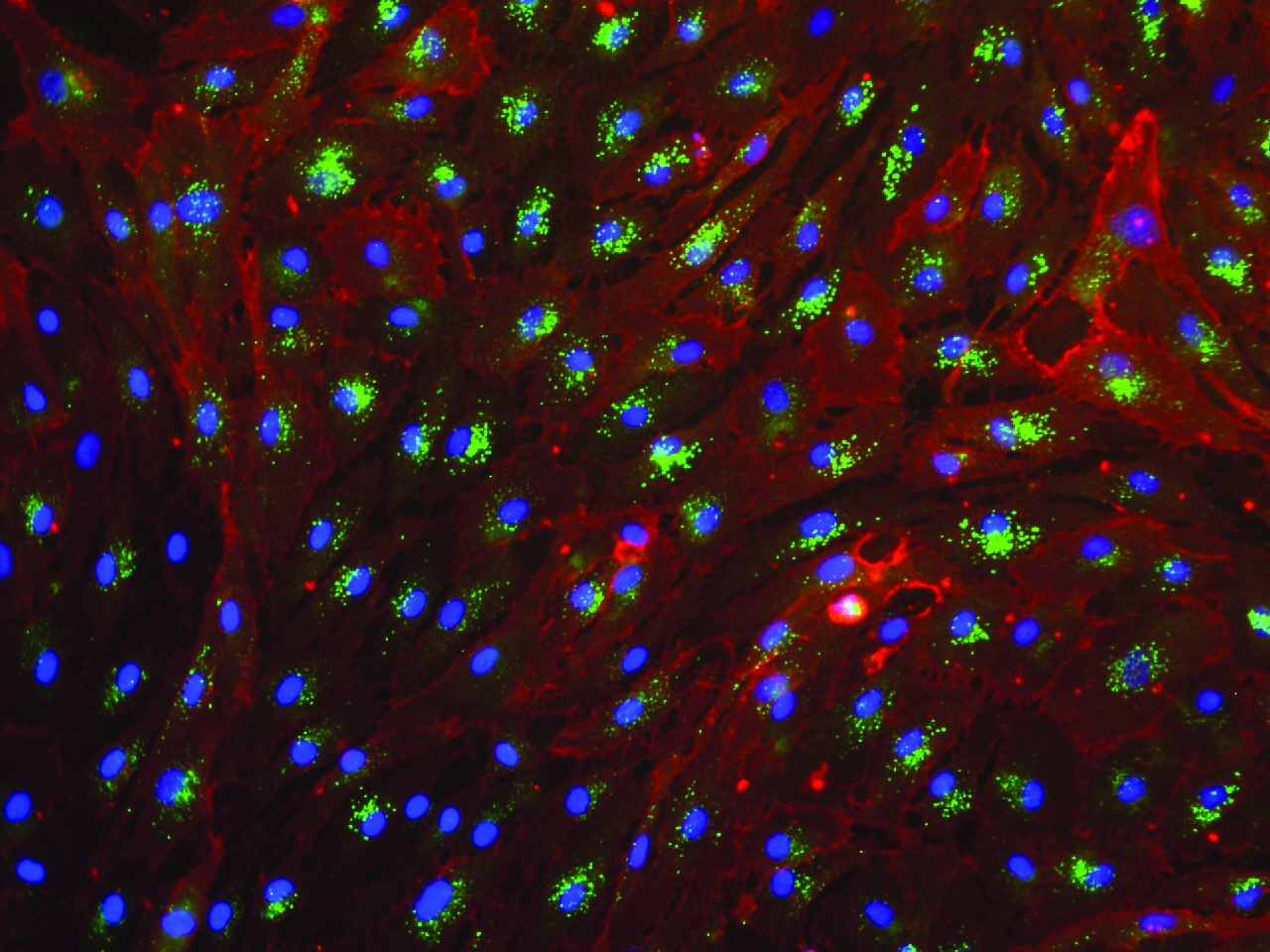
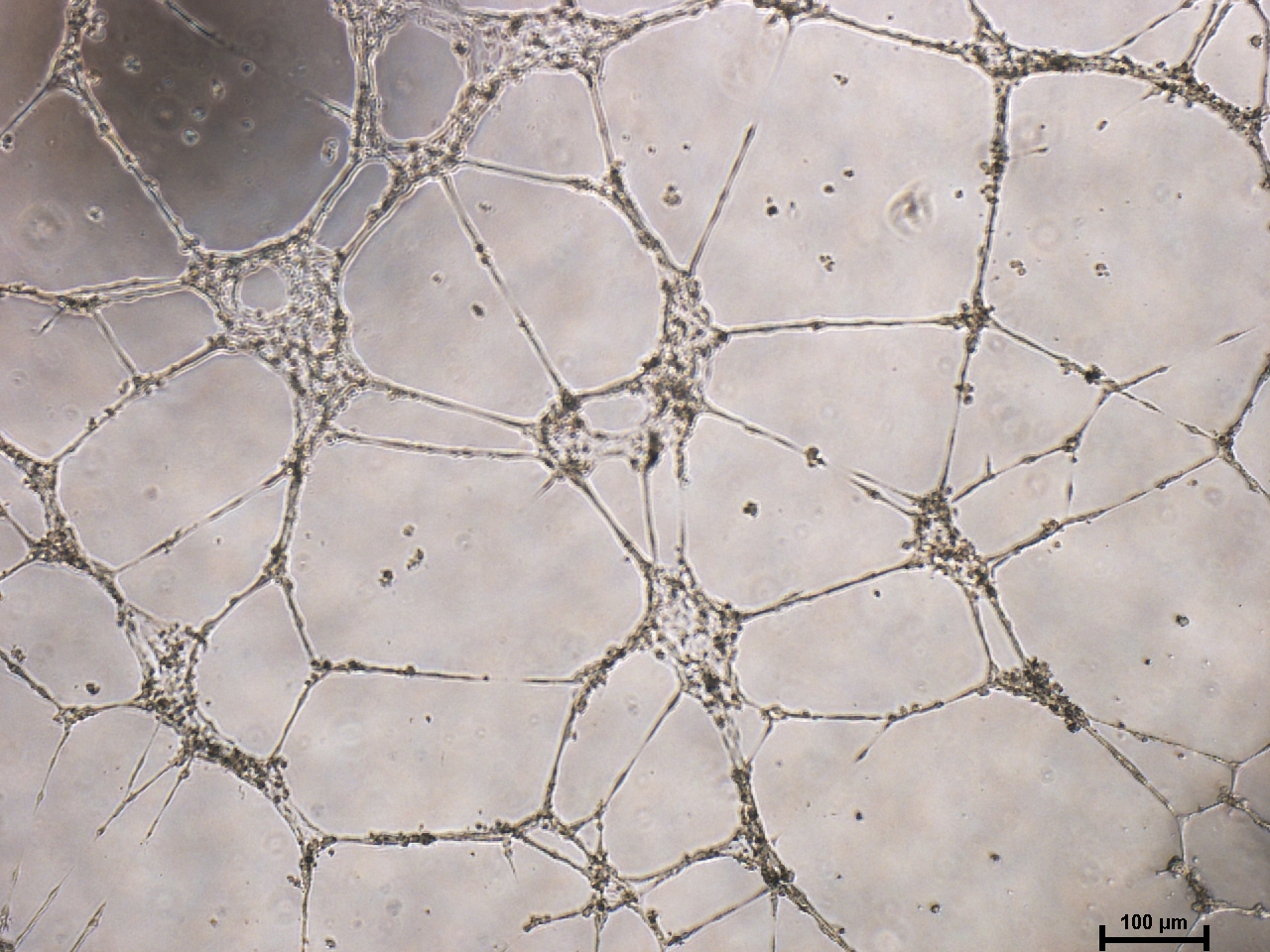
Microvascular endothalial cells and macrovascular endothelial cells maintain classic cobblestone like morphology after expansion for several sequential passages with equal seeding (5000 cells/cm2) in EndoGo™ XF +2% OTC human AB serum on hFN pre-coated dishes (A), expanded cells preserved endothelial cell features (EC markers expression) (B) and angiogenic potential to form capillary-like tubes (C).
Specifications
Specifications
| QTY | 500 mL |
|---|---|
| Instructions for Use | The complete endothelial cell culture media consist of two components: EndoGo™ XF Medium (05-400-1) and EndoGo™ XF Supplement Mix (05-410-1). Individual components are not sold separately. In addition, the medium required the use of human AB serum (off the clot) or human platelet lysate (not supplied with the media). Storage and Stability
|
References
references
- A.Ogunye et. al., Ex vivo expansion of cord blood-derived endothelial cells using a novel xeno-free culture media National Center for Biotechnology Information, U.S. National Library of Medicine, 2019 Mar 22;5(5):FSO376. doi: 10.2144/fsoa-2018-0103
- V. Alonso-Camino and W. Mirsch, In vitro expansion of human primary endothelial cells for clinical use using EndoGo™ XF Medium supplemented with PLTGold® human platelet lysate. Cytotherapy, May 2018Volume 20, Issue 5, Supplement, Page S87
Documentation
Materials Safety Data Sheet
Manuals and Protocols
 EndoGo™ XF Medium Instructions for Use
EndoGo™ XF Medium Instructions for Use POSTER: Cell Therapy Compliant Xeno-Free Culture System for Human Endothelial Cells
POSTER: Cell Therapy Compliant Xeno-Free Culture System for Human Endothelial Cells
Product Literature
Certificate of Analysis
COA's can be downloaded from Sartorius's Certificates Portal.
For certificates issued before November 15, 2021, please enter below the product lot number and click search.