Description
Details
Product Overview
MSCgo™ Chondrogenic Differentiation Medium is a serum-free (SF) and xeno-free (XF) formulation developed for optimal differentiation of human mesenchymal stem cells (hMSC) to mature chondrocytes. The MSCgo™ Chondrogenic Differentiation Medium is validated to efficiently differeniate hMSC from a variety of sources, including bone marrow (BM-MSC), adipose tissue (AT-MSC), and umbilical cord tissue (UC-MSC).
The MSCgo™ Chondrogenic Differentiation Medium kit includes a basal medium and supplement, containing all growth factors and supplements necessary for a complete chondrogenic differentiation medium. No additional supplements are required.
The MSCgo™ Chondrogenic Differentiation protocol is part of a complete system for multipotency evaluation of hMSCs. This kit enables reliable differentiation of hMSCs to mature chondrocytes without background differentiation or interruption in cellular metabolism.
Features
- Serum-free, xeno-free medium
- All required growth factors and supplements included in kit
- Reliable differentiation to mature chondrocytes
- Each lot is application tested
- Does not contain antibiotics
Components
- MSCgo Chondrogenic Differentiation Basal Medium: 100 mL
- MSCgo Chondrogenic Differentiation Supplement Mix : 10 mL
Chondrogenesis Results
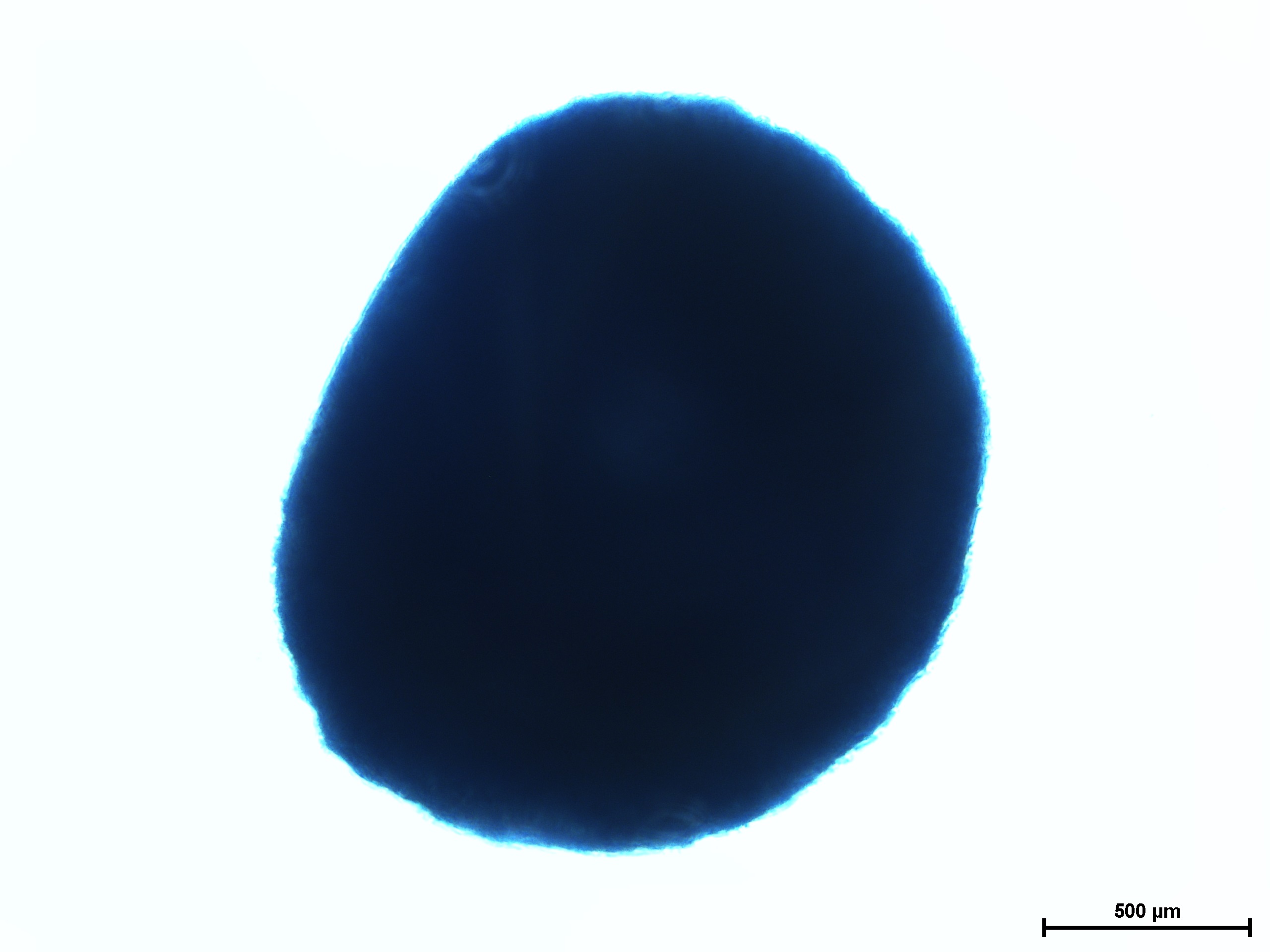
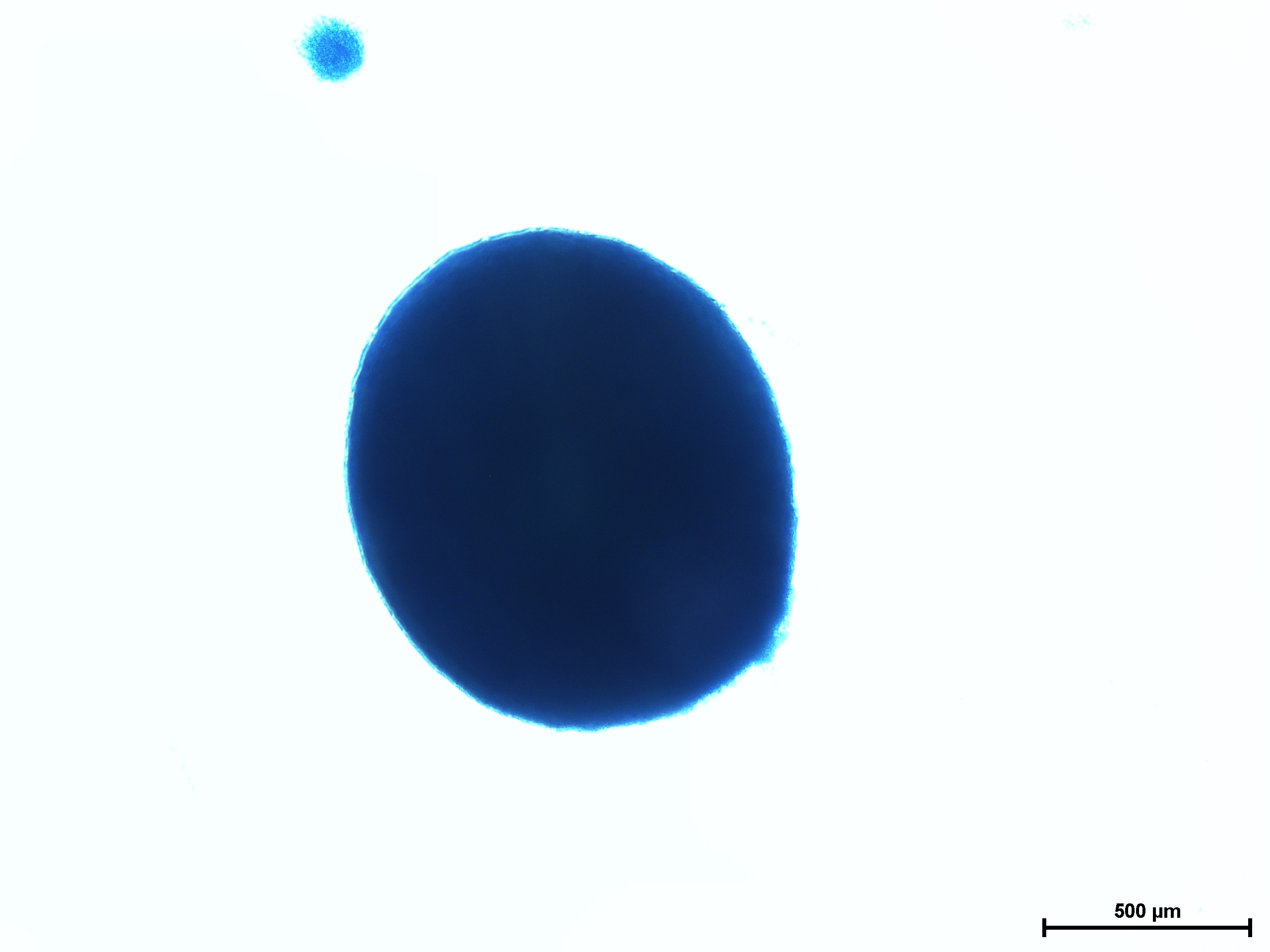
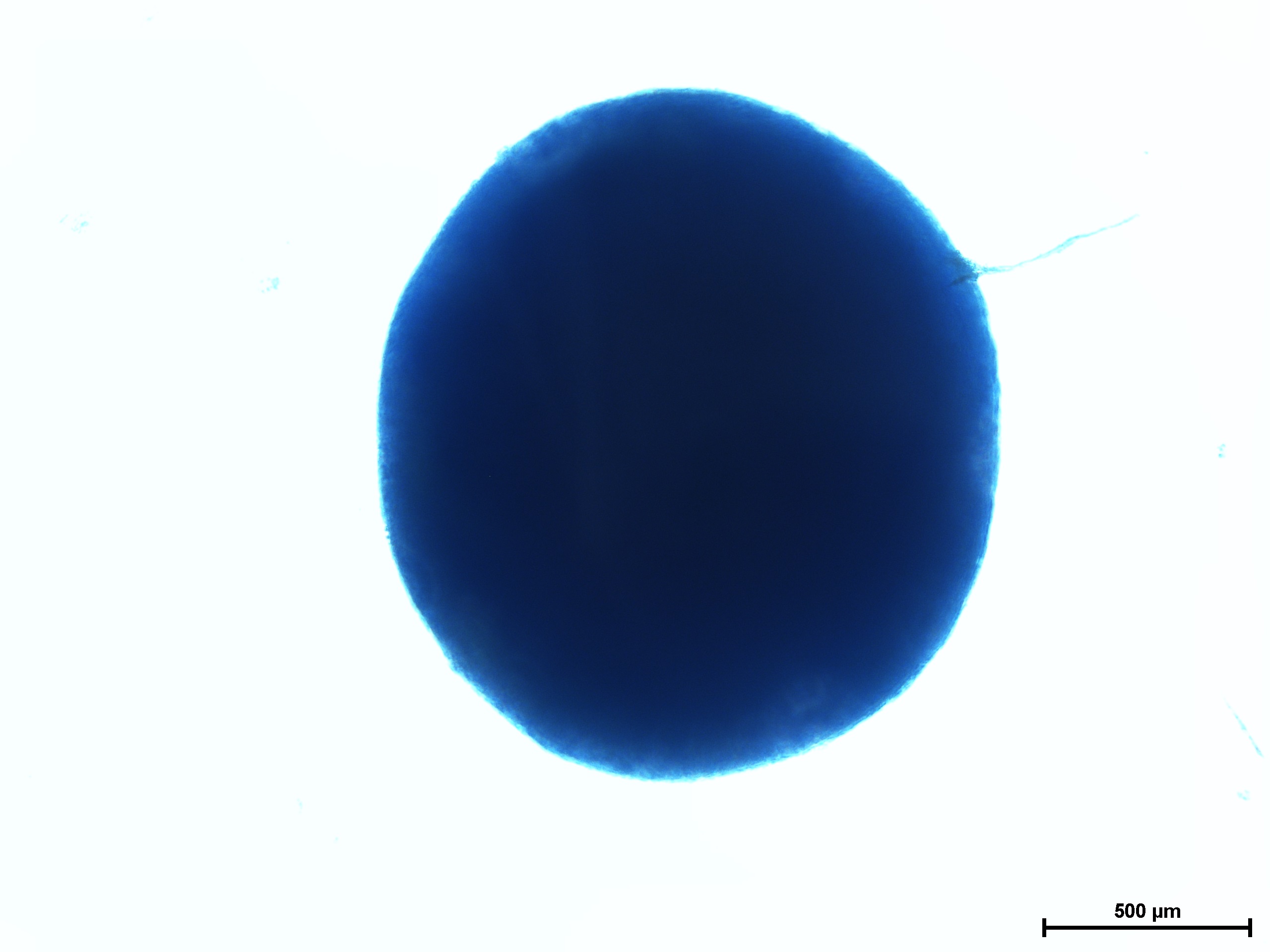
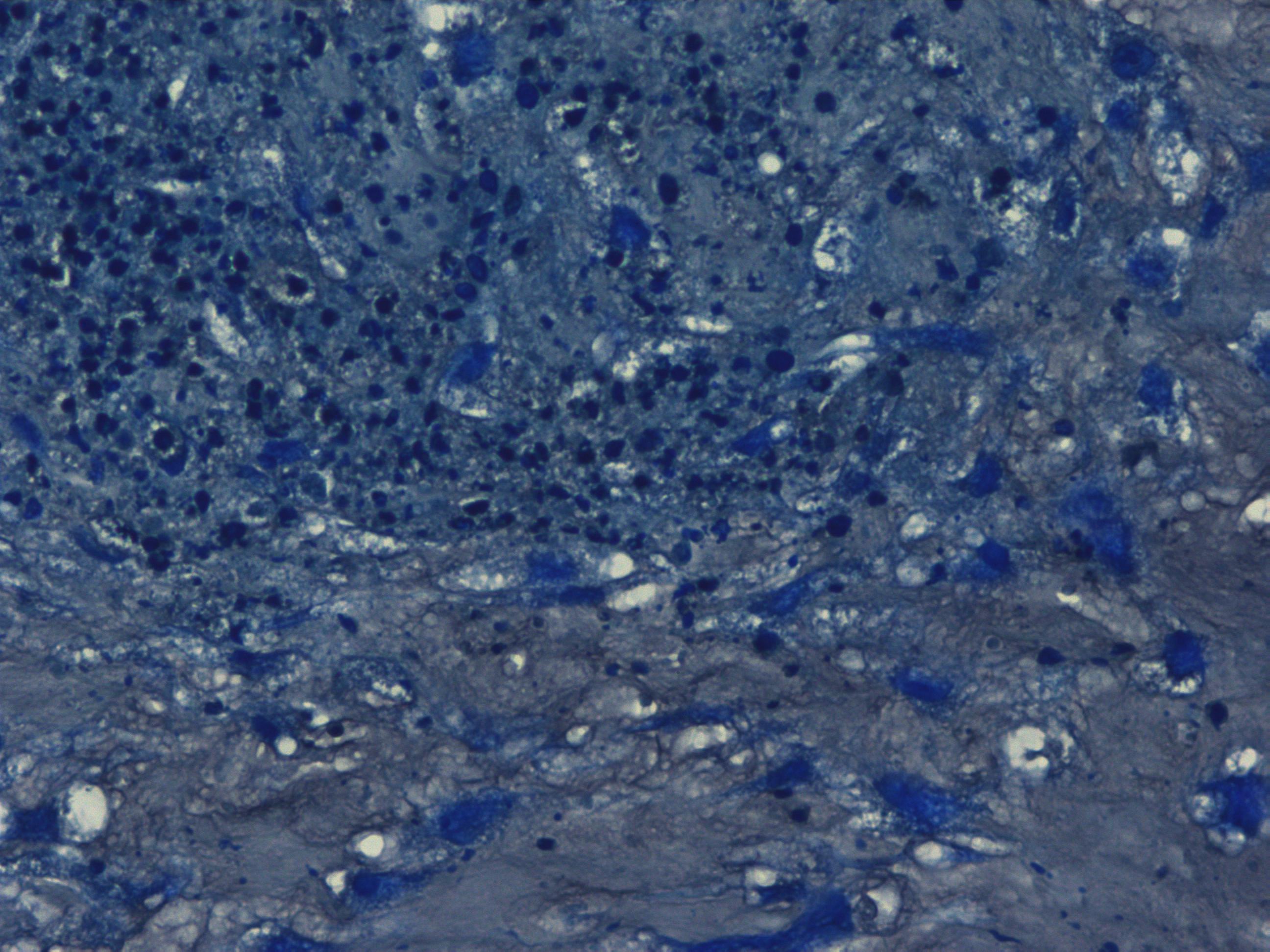
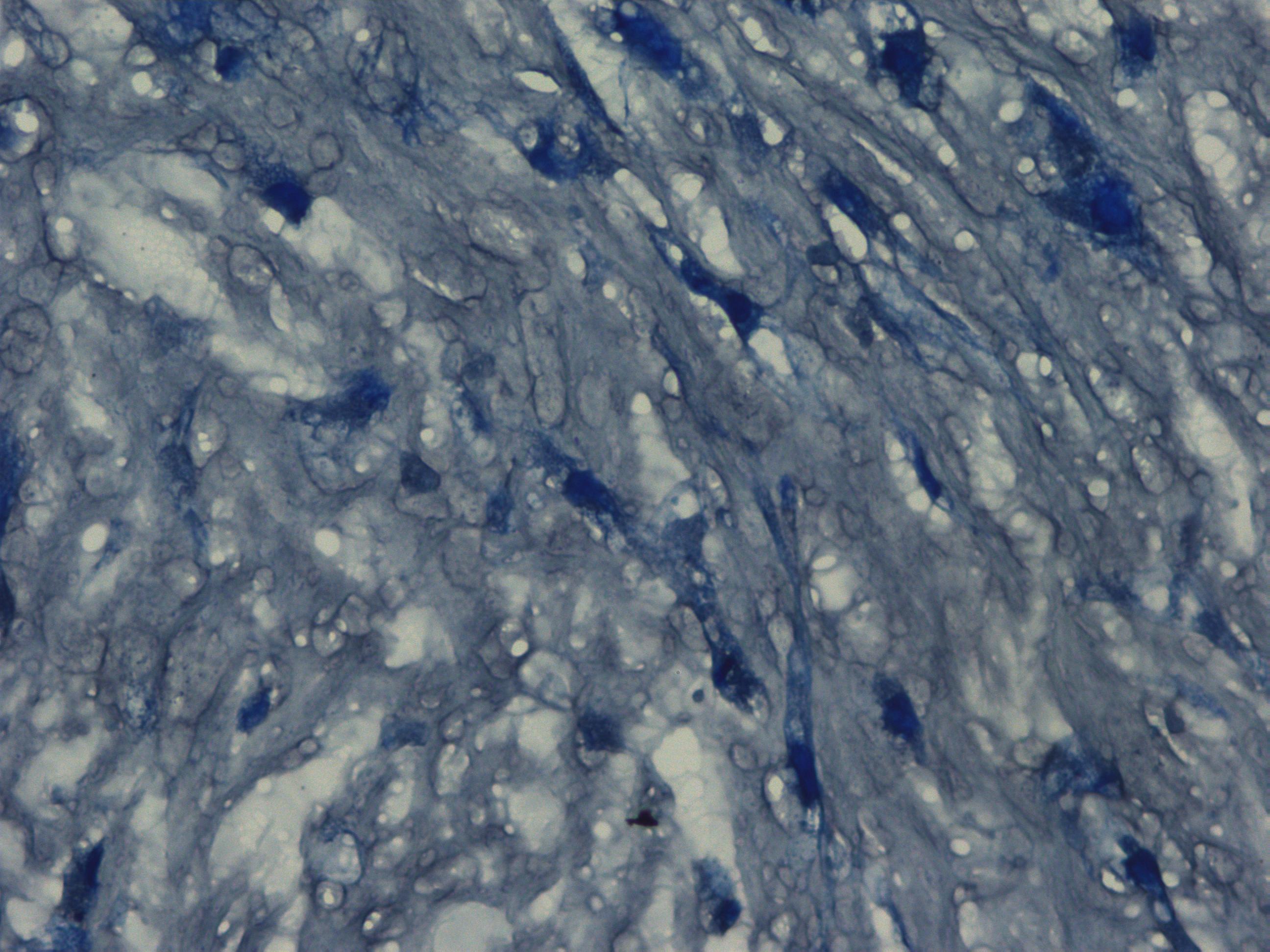
Chondrogenic differentiation of hMSC in 3D spheroid culture in the formation of cartilage with a typical extracellular matrix rich of Aggrecan. Aggrecan is a proteoglycan that can be used as an indicator for cartilage formation and can be detected with Alcian Blue, a dark-blue copper-containing dye that acts as an indication of mature chondrocytes. The staining intensity can be vary using different hMSC (e.g. source, age, and passage number).
Cartilage differentiation results of hMSC from various sources after 21 day assay using MSCgo™ Chondrogenic followed by Alcian Blue staining.
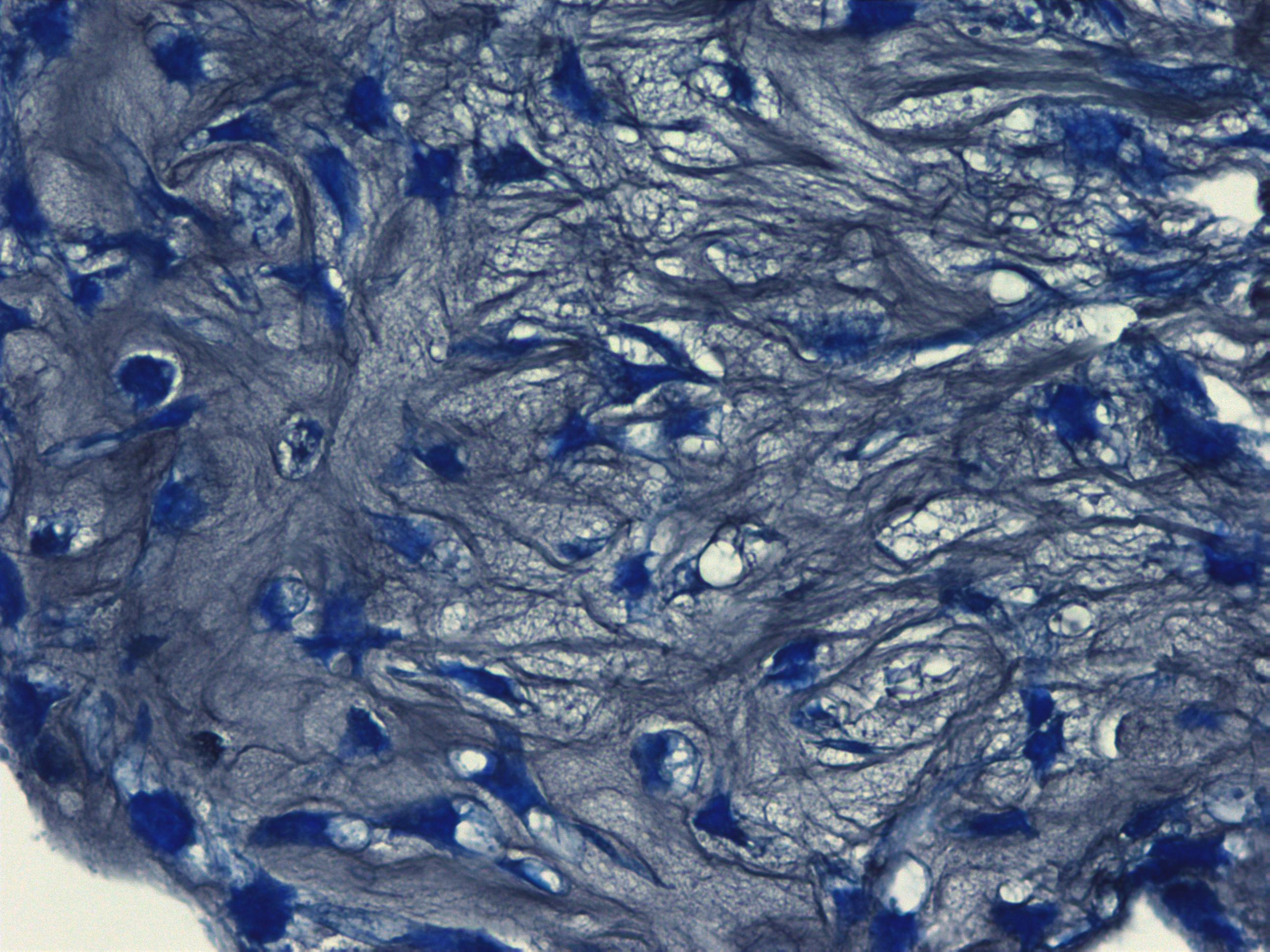
Histological images of mature chondrocytes surrounded by a cartilage matrix after a 21-day differentiation assay using MSCgo™ Chondrogenic Medium followed by Toluidine blue (40x).
Specifications
Specifications
| Form | Liquid |
|---|---|
| Brand | MSCgo™ |
| Storage Conditions | Chondrogenic Differentiation Basal Medium: 2 to 8°C Chondrogenic Differentiation Supplement Mix : -20°C |
| Quality Control | The MSCgo Chondrogenic Differentiation Kit is validated for optimal differentiation of hMSC into chondroocytes. Additional tests are: pH, osmolality, endotoxins and sterility tests. |
| Specifications | Required Materials for Chondrogenic Assay
|
| Instructions for Use | Complete Medium Preparation
Note: The combination of the MSCgo Chondrogenic Supplement Mix into the Basal Medium generates a complete medium that is ready for use. No additional supplements are required. |
| Legal | For human ex vivo tissue and cell culture processing applications. This reagent is not approved for human or animal use, or for application of in vitro diagnostic procedures. |
References
references
- C. Zheng et al. miR-92a-3p-inspired shRNA exhibits pro-chondrogenic and chondrocyte protective effects in osteoarthritis treatment through targeting SMAD6/7. Journal of Bone and Mineral Metabolism, 2023
- L. Meesuk et al. Osteogenic differentiation and proliferation potentials of human bone marrow and umbilical cord-derived mesenchymal stem cells on the 3D-printed hydroxyapatite scaffolds. Scientific Reports, 2022
- W. Zhou et al. Autophagy inhibition restores CD200 expression under IL-1β microenvironment in placental mesenchymal stem cells of fetal origin and improves its pulmonary fibrosis therapeutic potential. Molecular Immunology, Volume 151, November 2022
- V.P. Mantripragada and G.F. Muschler Improved biological performance of human cartilage-derived progenitors in platelet lysate xenofree media in comparison to fetal bovine serum media. Current Research in Translational Medicine, 2022
- S. Cai et al. Single-cell RNA sequencing reveals the potential mechanism of heterogeneity of immunomodulatory properties of foreskin and umbilical cord mesenchymal stromal cells. Cell & Bioscience volume 12, Article number: 115 (2022)
- X. Kang et al. . Zuogui Wan slowed senescence of bone marrow mesenchymal stem cells by suppressing Wnt/β-catenin signaling. Journal of Ethnopharmacology, 2022
- X. Zhang, et al. Human umbilical cord mesenchymal stem cell-derived exosomal microRNA-148a-3p inhibits neointimal hyperplasia by targeting Serpine1. Archives of Biochemistry and Biophysics, Volume 719, 2022
- V.A. Nikitina, et al. Cytogenetic Characteristics of Diploid Lines of Mesenchymal Multipotent Stromal Cells. Cell Tiss. Biol. 15, 604–615 (2021). https://doi.org/10.1134/S1990519X21060146
- J. Wang, et al. Trehalose glycopolymers for cryopreservation of tissue-engineered constructs. Cryobiology, 2021, https://doi.org/10.1016/j.cryobiol.2021.11.004.
- S. Horikoshi, et al. Clumps of Mesenchymal Stem Cells/Extracellular Matrix Complexes Generated with Xeno-Free Chondro-Inductive Medium induce Bone Regeneration via Endochondral Ossification. Biomedicines 2021, https://doi.org/10.3390/ biomedicines9101408
- C. Siyu, et al. Single Cell Transcriptome Sequencing Reveals the Potential Mechanism of Heterogeneity in Immunoregulatory Function Between Mesenchymal Stromal Cells. research square 2021, DOI: https://doi.org/10.21203/rs.3.rs-823639/v1
- Y. Matsuo, et al. Isolation of adipose tissue-derived stem cells by direct membrane migration and expansion for clinical application. Human Cell (2021). https://doi.org/10.1007/s13577-021-00505-3
- S. Kikuchi, et al. Development of a nasal mucosa-removal model for evaluating cell therapy. Regenerative Therapy, Volume 16, 2021, P. 32-41, ISSN 2352-3204, https://doi.org/10.1016/j.reth.2020.12.004.
- J. Min, et al. Phenotype and biological characteristics of endometrial mesenchymal stem / stromal cells:A comparison between intrauterine adhesion patients and healthy women. AJRI, 18 November 2020, https://doi.org/10.1111/aji.13379
- E. Çerçi & H. Erdost, Rapid, practical and safe isolation of adipose derived stem cells. Biotechnic & Histochemistry, 23 Jun 2020. DOI: 10.1080/10520295.2020.1776895
- E. Çerci & H.Erdost, Stem Cell Identification and Clinical Practice International Journal of Agricultural and Natural Sciences E-ISSN:2651-3617 12(1): 17-19, 2019
- O. Ben Menachem - Zidon, et al. Systemically transplanted mesenchymal stem cells induce vascular-like structure formation in a rat model of vaginal injury plos one, June 13, 2019 https://doi.org/10.1371/journal.pone.0218081
- M.Y. Meng et. al. Assessment of tumor promoting effects of amniotic and umbilical cord mesenchymal stem cells in vitro and in vivo. J Cancer Res Clin Oncol (2019) 145: 1133. https://doi.org/10.1007/s00432-019-02859-6
- R. Moloudi et al. Inertial-Based Filtration Method for Removal of Microcarriers from Mesenchymal Stem Cell Suspensions. Scientific Reportsvolume 8, Article number: 12481 (2018)
- J. Leber et al., Microcarrier choice and bead-to-bead transfer for human mesenchymal stem cells in serum-containing and chemically defined media. Process Biochemistry, volume 59, Part B, August 2017, Pages 255-265
- L. Pu et al., Compared to the amniotic membrane, Wharton’s jelly may be a more suitable source of mesenchymal stem cells for cardiovascular tissue engineering and clinical regeneration. Stem Cell Research & Therapy 2017 8:72
Documentation
Materials Safety Data Sheet
 MSCgo™ Chondrogenic Differentiation Medium GB (MSDS)
MSCgo™ Chondrogenic Differentiation Medium GB (MSDS) MSCgo™ Chondrogenic Differentiation Medium US (MSDS)
MSCgo™ Chondrogenic Differentiation Medium US (MSDS) MSCgo™ Chondrogenic Differentiation Supplement Mix GB (MSDS)
MSCgo™ Chondrogenic Differentiation Supplement Mix GB (MSDS) MSCgo™ Chondrogenic Differentiation Supplement Mix US (MSDS)
MSCgo™ Chondrogenic Differentiation Supplement Mix US (MSDS)
Manuals and Protocols
Product Literature
Certificate of Analysis
COA's can be downloaded from Sartorius's Certificates Portal.
For certificates issued before November 15, 2021, please enter below the product lot number and click search.