Description
Details
Product Overview
Vitronectin XF™ is a defined, xeno-free cell attachment factor intended to support the growth and differentiation of human pluripotent stem cells (hPSCs) under serum-free, feeder-free conditions. Vitronectin XF™ is developed and manufactured by Nucleus Biologics, Inc. and distributed by Biological Industries USA, Inc. Vitronectin XF™ is a recombinant fusion protein that contains the entire human Vitronectin sequence (NCBI Reference Seq. NP_000629.3), and is to be used for coating of sterile, non-tissue culture-treated polystyrene dishes and plates. When used in combination with NutriStem® hPSC Medium, Vitronectin XF™ supports long-term growth and expansion of hPSCs in a completely defined, xeno-free culture system.
Vitronectin XF™ maintains pluripotency and normal karyotype throughout long-term culture of hPSCs in defined conditions without the need for a ROCK inhibitor when used in combination with defined, xeno-free NutriStem® hPSC media.
Vitronectin is a consistent and effective single-component alternative to Matrigel® and other undefined matrices, with simple plating protocols and enzyme-free passaging options.
Sample Data
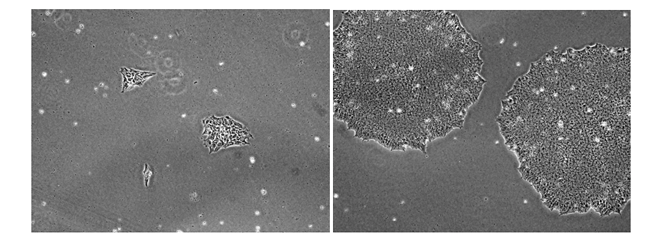
Figure 1: Normal cell morphology. hiPS cells thawed directly into NutriStem® hPSC XF Medium on Vitronectin XFTM substrate in the absence of the ROCK inhibitor Y-27632. The images demonstrate that cells grown in these conditions assume normal pluripotent cell morphologies as they expand from the early (Day 1, left image) to the late (Day 7, right image) phases of growth after thawing and/or passaging. Images shown at 20X magnification.
Advantages
- Defined, xeno-free cell attachment substrate
- Full-length recombinant human vitronectin protein
- Simple plating protocol
- Supports enzyme-free passaging and reproducible long-term hPSC culture
- Cost-effective alternative to Matrigel®
- Validated in combination with NutriStem® hPSC Medium
- Effectively supports both hPSC maintenance and differentiation
Specifications
Specifications
| QTY | 2 mL / 500 µg |
|---|---|
| Form | Liquid |
| Storage Conditions | Store undiluted at -20° to -80°C, protected from light, for up to 12 months. Thaw vial at room temperature. Once thawed, store undiluted at 4°C, protected from light, for up to 2 weeks. Product is stable for a minimum of 6 months from date of manufacture |
| Specifications | Designations: Xeno-free; defined Concentration: 250 µg/mL Source: Liquid Sterility: 0.2 µm Sterile filtered; Mycoplasma-negative Authenticity: Verified by N-terminal and mass spectrometry analyses Purity: > 90% pure by SDS-PAGE gel and Coomassie Blue staining Protein Content: Verified by UV spectroscopy and/or SDS-PAGE gel Endotoxin: < 1.25 EU/mL Biological Activity: Pluripotent hiPSC culture (> 5 passages) and differentiation into definitive endoderm |
| Instructions for Use |
|
| Legal | For research use only.Vitronectin XF™ is developed and manufactured by Primorigen Biosciences, Inc. Primorigen® and Primorigen Biosciences® are registered trademarks of Primorigen Biosciences. Vitronectin XF™ is a trademark of Primorigen Biosciences. |
Documentation
Protocols
Product Literature
 Vitronectin - Product Information Sheet
Vitronectin - Product Information Sheet Case Study: Xeno-Free hiPS Cell Culture using NutriStem® hPSC XF Medium on a Human Vitronectin Substrate without a need for ROCK Inhibitors
Case Study: Xeno-Free hiPS Cell Culture using NutriStem® hPSC XF Medium on a Human Vitronectin Substrate without a need for ROCK Inhibitors Technical Brief: Passaging methods
for hPSCs under feeder-free conditions
Technical Brief: Passaging methods
for hPSCs under feeder-free conditions Blog Article: Observations from the Field: Efficient hiPS cell culturing without ROCK inhibitors
Blog Article: Observations from the Field: Efficient hiPS cell culturing without ROCK inhibitors
Certificate of Analysis
COA's can be downloaded from Sartorius's Certificates Portal.
For certificates issued before November 15, 2021, please enter below the product lot number and click search.







